Bayer HealthCare Pharmaceuticals Inc and Onyx Pharmaceuticals, Inc. today announced that a Supplemental New Drug Application for Nexavar
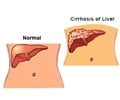
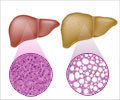
Bayer HealthCare Pharmaceuticals Inc. (NYSE: BAY) and Onyx Pharmaceuticals, Inc. (Nasdaq: ONXX) today announced that a Supplemental New Drug Application (sNDA) for Nexavar(R) (sorafenib) tablets has been submitted to the U.S. Food and Drug Administration (FDA) for the treatment of patients with hepatocellular carcinoma (HCC), the most common form of liver cancer.
Nexavar is currently approved in more than 50 countries for the treatment of advanced kidney cancer. The companies also confirmed that they are planning a company- sponsored Phase 3 study of Nexavar in the adjuvant treatment of HCC following the complete removal of early stage liver cancer.The sNDA submission is based on positive data from the international, Phase 3, placebo-controlled Sorafenib HCC Assessment Randomized Protocol (SHARP) trial which demonstrated that Nexavar extended overall survival by 44 percent in patients with HCC (HR=0.69; p=0.0006) versus placebo.
There were no significant differences in serious adverse event rates between the Nexavar and placebo-treated groups with the most commonly observed adverse events in patients receiving Nexavar being diarrhea and hand-foot skin reaction. Currently, there are no FDA-approved drug therapies that significantly extend survival of patients with liver cancer.
"These results are particularly meaningful considering that death rates from liver cancer continue to increase," said Susan Kelley, M.D., vice president, Therapeutic Area Oncology, Bayer HealthCare Pharmaceuticals. "After more than 100 clinical studies of many agents over three decades.
Nexavar is the first drug therapy to demonstrate a significant survival benefit for patients with HCC, and, if approved, may fulfill a serious unmet need with a manageable toxicity profile."
HCC, the most common form of liver cancer, is responsible for about 90 percent of the primary liver cancers in adults. It is the fifth most common cancer in the world and the third leading cause of cancer-related deaths globally.
Advertisement
"This filing exemplifies our commitment to providing valuable therapeutic options for significant unmet needs in cancer treatment," said Hank Fuchs, M.D., executive vice president and chief medical officer of Onyx.
Advertisement
Source-PRNewsWire
MED