Two medical ethics experts have said that the limited doses of Ebola trial drugs must not be reserved for the well-off or well-connected.
"Especially in a dire emergency such as this one, well-off and well-connected patients should not be further privileged."
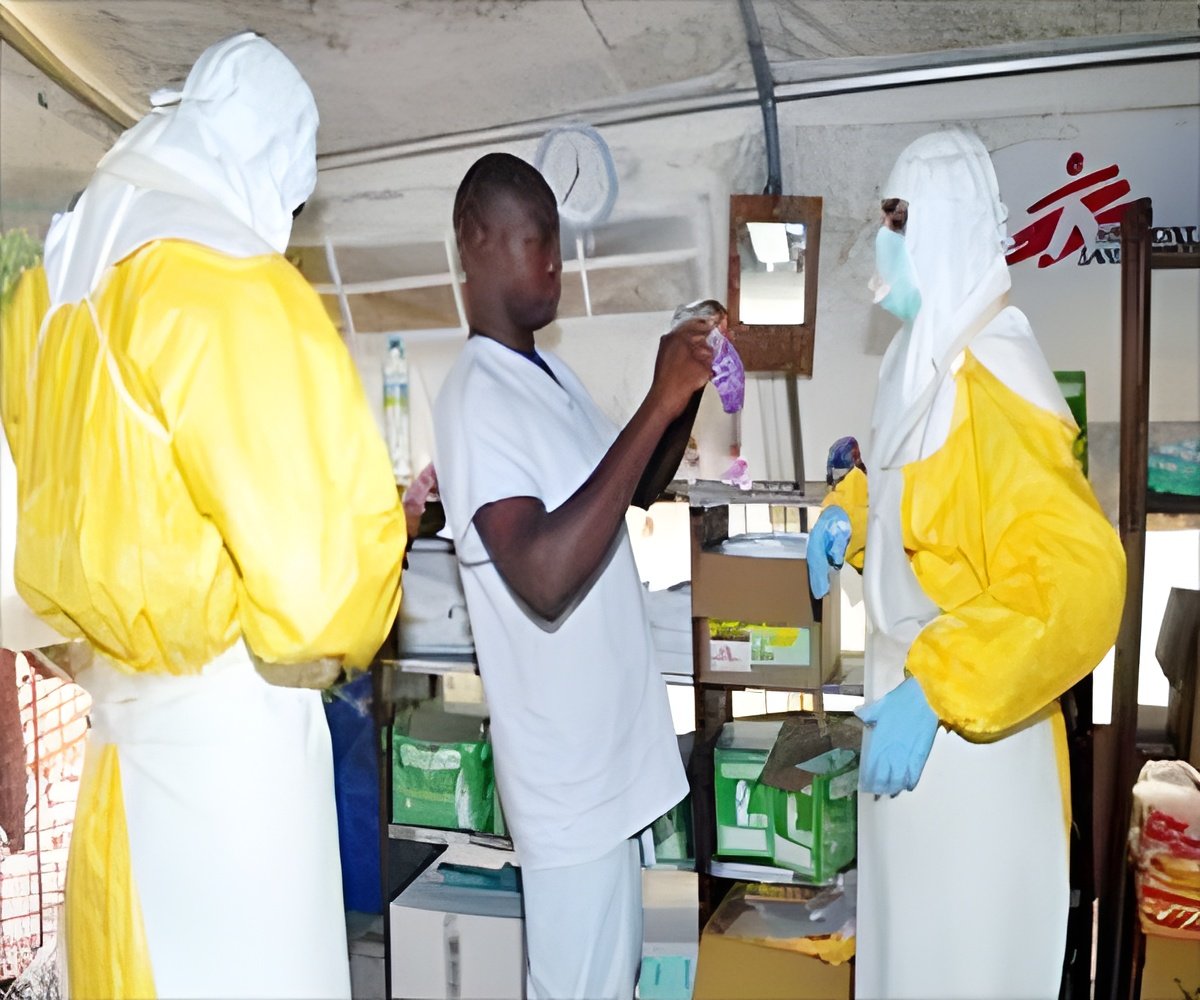
The pair said the limited supply of ZMapp, an experimental cocktail of three antibodies, has been given "almost exclusively to health-care workers".
And while some have argued this was correct since they were putting themselves at risk to help others, they could be seen as having an unfair advantage over other, local helpers.
"Health-care workers are often well-off and have special ties to the medical establishment," said Emanuel and Rid.
No approved drug exists, though several are under development and the World Health Organisation last week gave the green light for experimental medicines to be used in fighting the killer disease.
Advertisement
Brantly and Writebol, three doctors in Liberia and a Spanish priest are known to have been given the trial treatment for a tropical virus that has killed 1,350 people, roughly half the number infected.
Advertisement
Emanuel and Rid said a handful of doses of ZMapp known to exist "has already been exhausted", and it would "take months" to produce new stock.
On Wednesday, University of Oxford epidemiologist Oliver Brady calculated in the journal Nature that 30,000 people would have required Ebola drugs by now had they been available.
Emanuel and Rid stressed that containing the virus, not focusing on a treatment, would end the outbreak.
Less than 10 percent of all candidate drugs ever make it through testing to enter the market, they said.
"In other words, it is more likely than not that the interventions will not improve symptoms for patients, and might even weaken them as they battle a life-threatening disease."
But if experimental drugs are given, it should be done only in clinical trials "so that researchers can learn whether they work or not," wrote the pair.
Source-AFP