Beta-lactone inhibits mycomenbrane biosynthesis and increases the efficiency of antibiotics to kill the bacteria.
‘Beta lactone EZ120 inhibits the biosynthesis of the mycomembrane and kills mycobacteria effectively.’





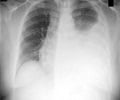
The mycomembrane of the tuberculosis pathogen Mycobacterium tuberculosis consists of a lipid double layer that encapsulates the cell wall, forming an exterior barrier. Structural hallmarks are mycolic acids, branched beta-hydroxy fatty acids with two long hydrocarbon chains. The team hypothesizes that similarly structured beta lactones could "mask" themselves as mycolic acid to enter the mycolic acid metabolic pathways and then block the decisive enzymes.
Helpful disrupter
In the context of an extensive search, the interdisciplinary team of scientists hit the bullseye with the beta lactone EZ120. It does indeed inhibit the biosynthesis of the mycomembrane and kills mycobacteria effectively.
Using enzyme assays and mass spectroscopy investigations, Dr. Johannes Lehmann, a researcher at the Chair of Organic Chemistry II at TU Munich, demonstrated during his doctoral work that the new inhibitor blocks especially the enzymes Pks13 and Ag85, which play a key role in the development of mycomembranes.
Advertisement
"The scientists suspect that disrupting the mycomembrane enables antibiotics to enter the bacteria more easily. This is a new mode of action and might be a starting point for novel tuberculosis therapies.
Advertisement
Source-Eurekalert