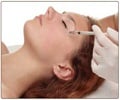
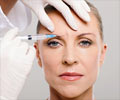
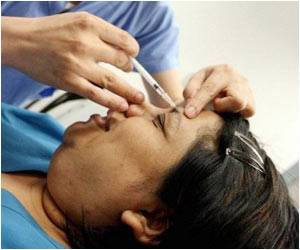
Months after US authorities sounded the alarm, European officials are warning of dangerous possible side effects from the wrinkle-smoothing injection Botox, according to a German news report.
A German news report has revealed that European officials have issued a warning of dangerous possible side effects from the wrinkle-smoothing injection Botox.
The London-based European Medicines Agency had by August 2007 recorded more than 600 cases of negative effects potentially linked to the popular cosmetic treatment, Focus news weekly reported in its issue to be released Monday.In 28 cases Botox users died.
In Germany the Federal Institute for Medication and Medical Products has received 210 reports with a suspected link to Botox, which is used by millions around the world to iron out wrinkles. Five cases were lethal, Focus said.
Botulinum toxins including Botox are approved in the United States and Europe to treat a variety of conditions including spasms of the eyelids or neck, the easing of facial lines or excessive sweating.
The US Food and Drug Administration (FDA) warned in February that using Botox can have serious side effects including death, but stopped short of banning them.
It stressed at the time that no patients who had used Botox for cosmetic purposes were among the fatalities, but urged vigilance.
Advertisement
When injected, tiny doses paralyse a muscle and prevent it from contracting for between four and six months -- ideal for temporarily eliminating worry lines but potentially deadly if it affects the wrong muscles, authorities said.
Source-AFP
RAS/L