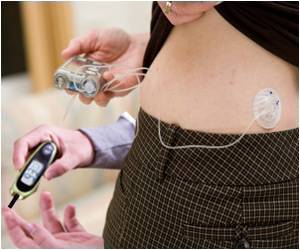
American researchers have successfully tested a bionic pancreas device, which imposes minimal restrictions on patient activities, in both adult and adolescents.
"In both of these studies this device far exceeded our expectations in terms of its ability to regulate glucose, prevent hypoglycemia and automatically adapt to the very different needs of adults – some of whom were very insulin-sensitive – and adolescents, who typically need higher insulin doses," says Edward Damiano, PhD, of the BU Department of Biomedical Engineering, principal investigator of the project and senior author of the NEJM report. "There's no current standard-of-care therapy that could match the results we saw."
Firas El-Khatib, PhD, of the BU Department of Biomedical Engineering, adds, "One of the key virtues of this device is its ability to start controlling the blood sugar instantly, based only on the patient's weight, and continually adapt its decision making regarding insulin and glucagon dosing to handle a wide range of dosing requirements." El-Khatib co-designed the bionic pancreas device with Damiano and is co-lead author of the NEJM report with Steven Russell, MD, PhD, of the MGH Diabetes Unit,.
Russell – who led the clinical trials reported in the current paper – Damiano, and El-Khatib previously published a 2010 Science Translational Medicine report that described successful use of the first-generation system in controlling the blood sugar of adults for 27 hours. But that study took place in a controlled hospital inpatient environment where participants essentially stayed in bed for the whole period and ate prescribed meals. "The key element with the current version of this device is that it's wearable, allowing participants to stay in something close to their usual environments, exercise and eat whatever they want," says Russell.
Additional inpatient trials conducted after the 2010 paper extended the study period to two days and included adolescents as well as adults. But developing a device that could be tested safely in an outpatient environment presented several challenges, first among which was a control system that could adapt not only to the minute-by-minute changing needs of an individual, but also to the very different needs of adults and adolescents. The rapid growth and hormonal changes of adolescence produces insulin requirements that are two to three times greater than those of adults of the same body weight, Damiano explains. And even though the dosage needs of adults are more predictable, contracting typical a illness like a cold or upset stomach can dramatically change the need for insulin over a period of days to weeks.
Advertisement