A new material that can be sutured into the hearts of infants who suffer from birth defects has been created by researchers at Rice University and Texas Children’s hospital.
The proof of their painstaking effort is in a petri dish in Jeffrey Jacot's lab, where a small slab of gelatinous material beats with the rhythm of a living heart.
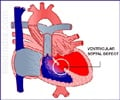
Patches used now to repair congenital heart defects are made of synthetic fabrics or are taken from cows or from the patient's own body. About one in 125 babies born in the United States suffers such a defect; three to six of every 10,000 have what's known as a defect called Tetralogy of Fallot, a cause of "blue baby syndrome" that requires the surgical placement of a patch across the heart's right ventricular outflow tract.
Current strategies work well until the patches, which do not grow with the patient, need to be replaced, said Jacot, an assistant professor of bioengineering at Rice University, director of the Pediatric Cardiac Bioengineering Laboratory at the Congenital Heart Surgery Service at Texas Children's Hospital and an adjunct professor at Baylor College of Medicine.
"None of those patches are alive," Jacot said, including the biologically derived patches that are "more like a plastic" and are not incorporated into the heart tissue.
"They're in a muscular area in the heart that's important for contraction and, more so, for electrical conduction," he said.
Advertisement
"What we're making can replace current patches in an operation that surgeons are already familiar with and that has a very high short- and medium-term success rate, but with long-term complications," he said.
Advertisement
The sandwich the researchers created seems to fill the bill on all counts. In the middle is a self-assembled polycaprolactone (PCL) polymer that hardens into a tough but stretchable ribbon. Mixing two types of PCL with different molecular weights allows tiny pores to form along the rough surface. The "bread" is a hydrogel made from a 50/50 mixture of gelatin and chitosan, a widely used material made from the shells of crustaceans like shrimp.
Heart cells cultured on the hydrogel surface were able to thrive and formed networks and ultimately beat. Though cells could not attach to the surface or pass through the pores of the PCL, the pores do allow nutrients to migrate from one side to the other, Jacot said.
They also allow the hydrogel to hold on to the PCL core.
The lab tested the biodegradable qualities of the PCL and found that over 50 days, about 15 percent dispersed, leaving a ragged sheet.
Years of testing await the researchers before human trials can begin, but Jacot and his team are already looking ahead to the possibilities their success could offer.
They hope to find a way to mix stem cell-derived heart cells from a patient into the hydrogel at the beginning of the process; stem cells may be drawn from several possible sources, including amniotic fluid routinely drawn from the newborn's mother, the subject of ongoing study by Jacot's lab.
The cells would make a patch genetically identical to the child that could be implanted shortly after birth.
"If we can make a patch that works immediately," Jacot said, "one that contracts and conducts and has living cells and grows with the patient, what other surgeries can we do that nobody can do now?"
Jacot, lead author Seokwon Pok, a postdoctoral researcher at Rice, and their tissue-engineering colleagues published their results in the Elsevier journal Acta Biomaterialia.
Source-ANI