The drug developed by the pharmaceutical company Hetero has been approved by the Drug Controller General of India.
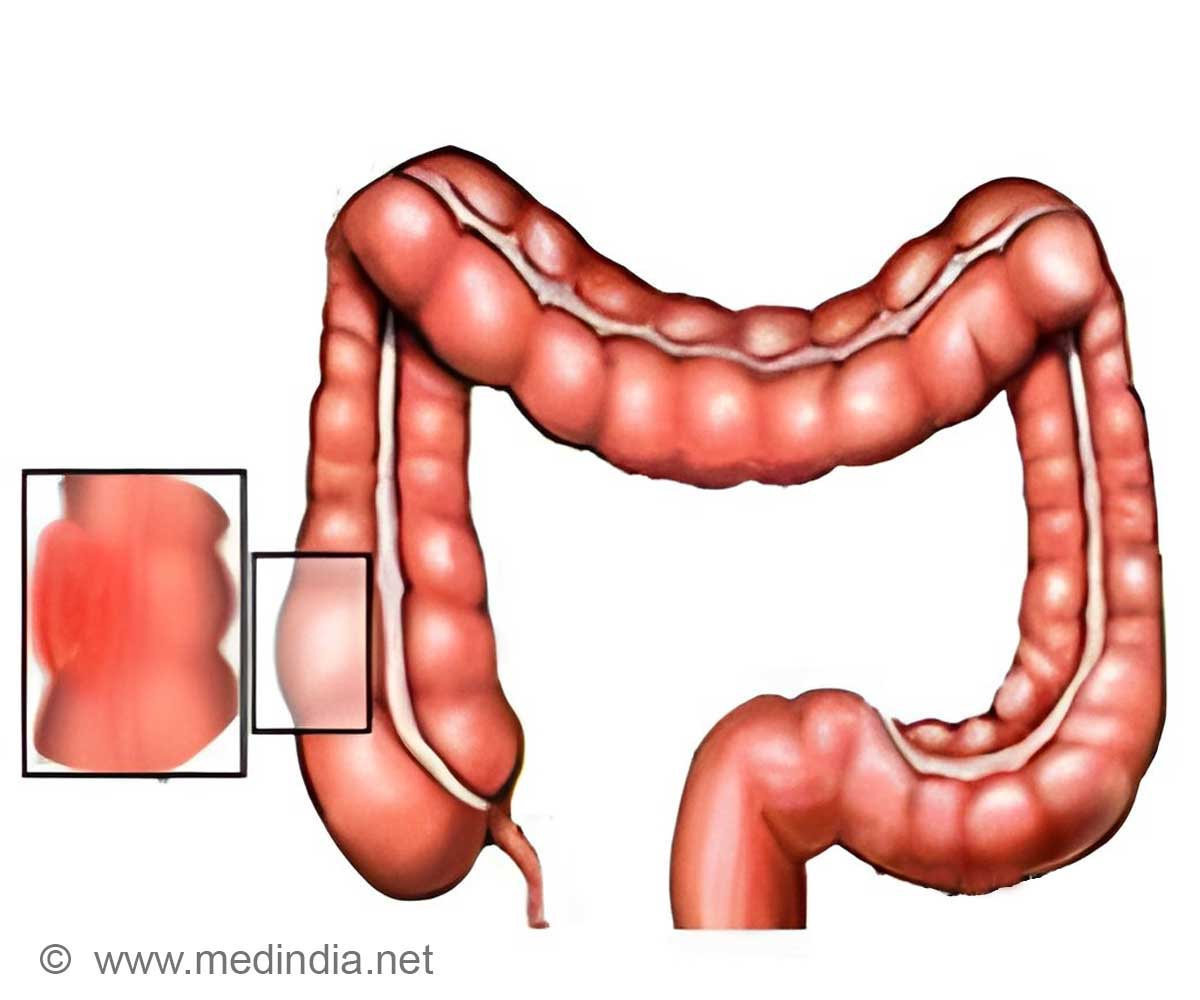
‘Hyderabad-based pharmaceutical company Hetero Drugs has launched its biosimilar 'Bevacizumab' for the treatment of metastatic colorectal cancer in India.
’





The product will be available in a single dose vial with two strengths, 100 and 400mg. Hetero will manufacture the drugs in the facility based in Hyderabad. Dr BPS Reddy, CMD, Hetero Group of Companies said: “It has been an exciting journey for us in Biologics. Hetero’s Bevacizumab is the third product in our biologics portfolio, after Darbepoetin alfa and Rituximab. We believe CizumabTM will be a cost-effective treatment option to patients in India.”
Source-Medindia