In patients with urothelial cancer of the bladder, immunotherapy after surgery helped to decrease cancer recurrence better than immunotherapy before surgery.
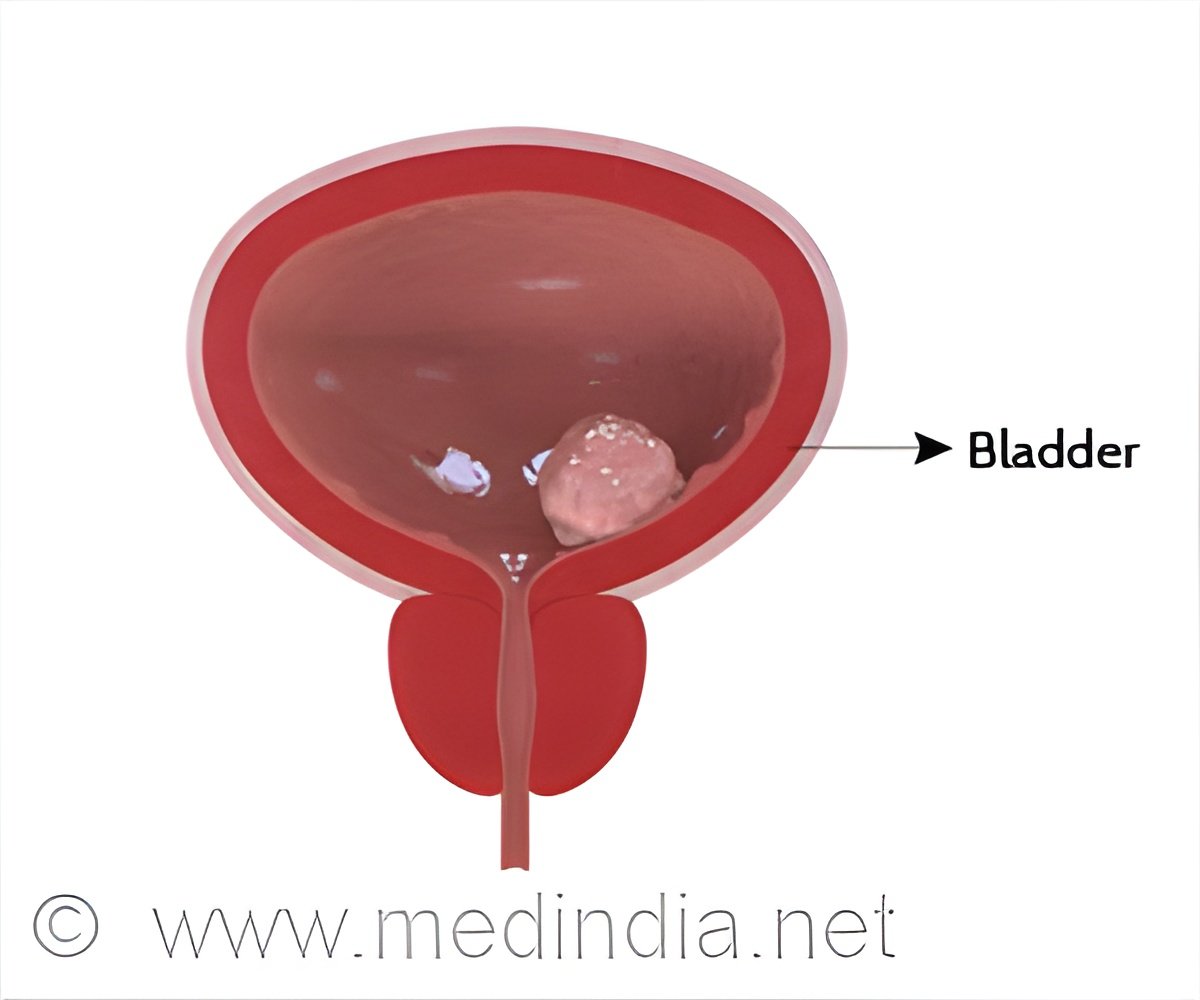
About 700 patients participated in phase 3, randomized, double-blind trial named CheckMate 274; half were given nivolumab and the other half placebo after having surgery with chemotherapy beforehand.
Immunotherapy Nivolumab for Bladder Cancer
“Longer-term follow-up data is important for reinforcing the initial results we published last year demonstrating for the first time that immunotherapy administered after surgery for bladder cancer and other urothelial cancer can decrease the risk of cancer recurrence,” said lead author and presenter Matthew Galsky, MD, director of Genitourinary Medical Oncology, Mount Sinai Tisch Cancer Center. “Almost 200,000 people die each year of urothelial cancer worldwide, so advances like immunotherapy being used in this manner bring hope.”Surgery that removes the bladder or kidney and ureter has been the standard of care for patients with urothelial cancer entering surrounding muscle or lymph nodes, but approximately half of these patients later relapse with lethal metastatic cancer.
Unfortunately for these patients, no consensus has emerged regarding treatments after surgery that might reduce the risk of cancer recurrence, so the results presented at AUA are essential.
In CheckMate 274, with a minimum of 11 months follow-up, patients who received nivolumab had an approximately 30 percent lower likelihood of developing cancer recurrence than those who received placebo.
Patients whose tumors had the gene PD-L1, making them more responsive to nivolumab’s cancer-fighting ability, and who received the immunotherapy had cancer-free rates that were even higher.
Advertisement
Source-Eurekalert