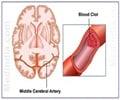
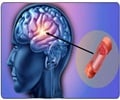
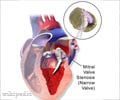
The US Food and Drug Administration has extended the approved indications for Bayer Healthcare’s blood clot drug, Xarelto
The drug, being developed by Bayer in collaboration with Johnson & Johnson, will now be used in preventing blood clots among patients with atrial fibrillation.
FDA’s director of cardiovascular and renal products division, Dr Norman Stockbridge said that doctors now have another option for treating patients who have high risk of developing blood clots. “This approval gives doctors and patients another treatment option for a condition that must be managed carefully”, he said.
Source-Medindia