Allergan, the US firm manufacturing the much vaunted Botox, is being slapped with compensation suits from those who say they have suffered heavily after Botox injections.
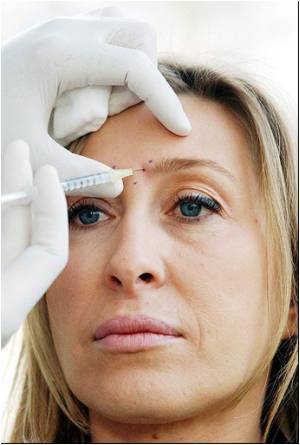
Dr. Sharla Helton, an obstetrician and gynecologist in Oklahoma said she fell ill and eventually lost her job after getting the injections in 2006.
She blamed Botox for double vision, breathing difficulty and years of continual pains in her arms, hands and feet.
Helton said the verdict was the “first step in making sure the public is aware of the actual risks of Botox. It's a steppingstone to protect the public from what the company is hiding.”
The jury found the company was negligent in its off-label promotion of the drug. Crucial testimony in the trial included an Allergan sales representative who admitted to providing off-label dosing guidelines to Botox clients in a sealed envelope, according to Helton's attorney, Ray Chester. However, the jury did not award punitive damages.
Allergan said it plans to appeal. The jury's negligence verdict “is inconsistent with all credible scientific and medical evidence,” company spokeswoman Caroline Van Hove said.
Advertisement
The trial began April 19 in district court in Oklahoma City and lasted more than three weeks, Orange County Register reported.
Advertisement
In the Spears case, Superior Court jurors in Santa Ana decided in March that Allergan was not responsible for the death of Spears' daughter Kristen, 7, a cerebral palsy patient who died in 2007 after Botox injections to relax her clenched muscles.
Allergan is seeking $460,000 in legal costs from Spears.
Chester said he hopes the Oklahoma verdict “will get the word out that people should be more careful about using Botox. Anyone who has been hurt by Botox may now have the courage to come forward,” he said.
Chester's next case relates to Sondra Bryant, a 70-year-old nurse who died in 2008 after receiving Botox injections for neck pain. The nurse was from Texas, but the trial will begin in Santa Ana, California, in the fall.
The US Food and Drug Administration (FDA) warned about potential risks associated with Botox in February 2008, after a number of reports were received involving deaths, breathing problems and other adverse reactions associated with use of Botox. At that time, the FDA indicated that the adverse reactions were most commonly seen among children with cerebral palsy, where the typical dose of Botox injection used is substantially larger than what is normally prescribed for cosmetic purposes.
In August 2009, the FDA announced a new “black box” warning about the risk of Botox problems, including potentially life-threatening botulism-like side effects. The warning now alerts users that the toxin used in Botox and other some other products could spread from the area of the injection to other areas of the body. This could cause botulism symptoms, including life-threatening swallowing and breathing difficulties or death.
Approved in the United States specifically to treat frown lines, crossed eyes and other conditions, Botox yields $1.3 billion in annual sales. One Wall Street analyst told the Wall Street Journal last year that as much as one-third of Botox sales are from uses for which it’s not been approved by the FDA. The drug uses botulinum toxin, a powerful poison, to block neural communications, allowing muscles that produce worry lines or gnarled limbs to relax. A few injections smooth wrinkles, while larger doses are required to relax arms and legs.
Allergan last October filed an unprecedent lawsuit, alleging the FDA ban on off-label marketing violates its First Amendment rights to free speech. The suit was filed a month after the FDA told Allergan to include a Risk Evaluation & Mitigation Strategies program to discuss some off-label prescribing guidelines with doctors. But to comply, Allergan argues it must tells doctors about the latest Botox data, even if some info involves off-label use - and that could prompt the government to file a lawsuit for off-label marketing.
Source-Medindia
GPL