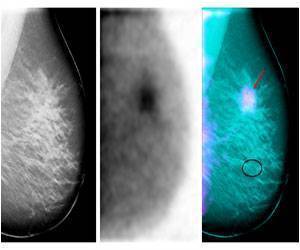
Tamoxifen is a highly effective drug that is used both to treat breast cancer and help prevent breast cancer in healthy women who are at high risk.
‘The study reassured women with breast cancer who fear endometrial side effects of the drug tamoxifen, as there was a very low rate of developing pre-malignant conditions.’





The study enrolled 296 eligible postmenopausal breast patients with a type of early-stage breast cancer called estrogen receptor-positive. Patients were randomly assigned to take tamoxifen alone or tamoxifen plus the hormone progestin. They were followed and assessed at years two and five. Researchers predicted that taking progestin would decrease the risk of abnormalities that can develop into uterine cancer. Such abnormalities occur in the endometrium (inner lining of the uterus).At year two, the primary time point, researchers evaluated 89 women who took tamoxifen alone and 80 women who took tamoxifen plus progestin. There were five endometrial abnormalities in the tamoxifen-alone group and one abnormality in the tamoxifen-plus-progestin group. This difference was not statistically significant. All abnormalities were benign. Only one additional benign abnormality was found at five years. No cancers were detected at two years or five years.
Two years after treatment began, only 6 percent of the patients in the tamoxifen-alone group, and 3.6 percent in both groups combined had benign endometrial abnormalities. Based on previous studies, researchers had projected that 30 percent of the women in the tamoxifen-alone group would show endometrial abnormalities.
Dr. Albain said the reason the abnormality rate was much lower than expected most likely was due to the study’s screening requirements. All women recruited for the study underwent uterine ultrasound exams before taking tamoxifen. If the ultrasounds showed a thickening of the inner lining of the uterus greater than 5 mm, a biopsy was performed. If the biopsy showed an endometrial abnormality, the woman was excluded from the study.
“For women who are concerned about taking tamoxifen to treat or prevent breast cancer, our study suggests that a normal ultrasound before treatment may provide additional reassurance,” Dr. Albain said.
Advertisement
“However, many women who would benefit from taking tamoxifen fail to do so because they fear getting endometrial cancer,” Dr. Potkul said. “Our study found that for women who did not have endometrial abnormalities when they began taking tamoxifen, there was a very low rate of developing pre-malignant conditions.”
Although there were more endometrial abnormalities in the tamoxifen-alone group, the difference was not large enough to be statistically significant. It would require a follow-up trial with thousands of women to determine whether adding progestin to tamoxifen truly reduces the risk of uterine cancer.
Researchers concluded, “These data could potentially reassure certain postmenopausal women who fear endometrial side effects of tamoxifen, but would otherwise benefit from instituting this important adjuvant breast cancer and breast cancer chemoprevention therapy. However, validation in a larger trial is needed first before changing practice in all asymptomatic postmenopausal women.”
Source-Newswise