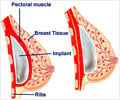
FDA, the top US drug agency warned women that while breast implants are safe, they are not meant to last a lifetime and could lead to problems later on.
The FDA approved the sale of silicone breast implants in November 2006 for women age 22 and above.
But in updated safety guidelines it warned that one in five women who have had breast implants to increase their cup-size or for reconstruction surgery will have to undergo another operation within 10 years.
"For patients who received implants for breast reconstruction, as many as one in two will require removal 10 years after implantation," the report said.
And one in five patients who received implants to boost their appearance will need them removed within 10 years.
Most complications occur from hardening of the breast tissue around the implant, from a rupturing of the implant, or "wrinkling, asymmetry, scarring, pain and infection," the FDA said.
Advertisement
The American Society of Plastic Surgeons said that last year 390,000 breast implant operations were carried out, the vast majority to increase bust size. Those figures include both silicone implants and those filled with saline water.
Advertisement
"Women who have enrolled in studies should continue to participate so that we may better understand the long-term performance of these implants and identify any potential problems."
Source-AFP