Learn why the FDA has mandated boxed warnings for CAR T-cell therapies, highlighting the risks of secondary malignancies.
- Secondary Primary Malignancy rates did not significantly differ among various CAR T-cell therapies or cancer types
- Patients with more than three prior lines of therapy had a higher SPM risk
- CAR T-cell therapy has a comparable risk of secondary cancers to standard treatments, with SPM rates of 5% versus 4.9%, respectively
Second primary malignancies after CAR T-cell therapy: A systematic review and meta-analysis of 5,517 lymphoma and myeloma patients
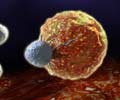
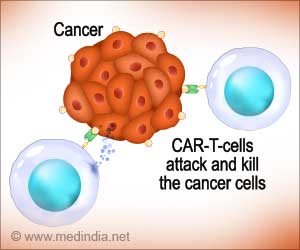
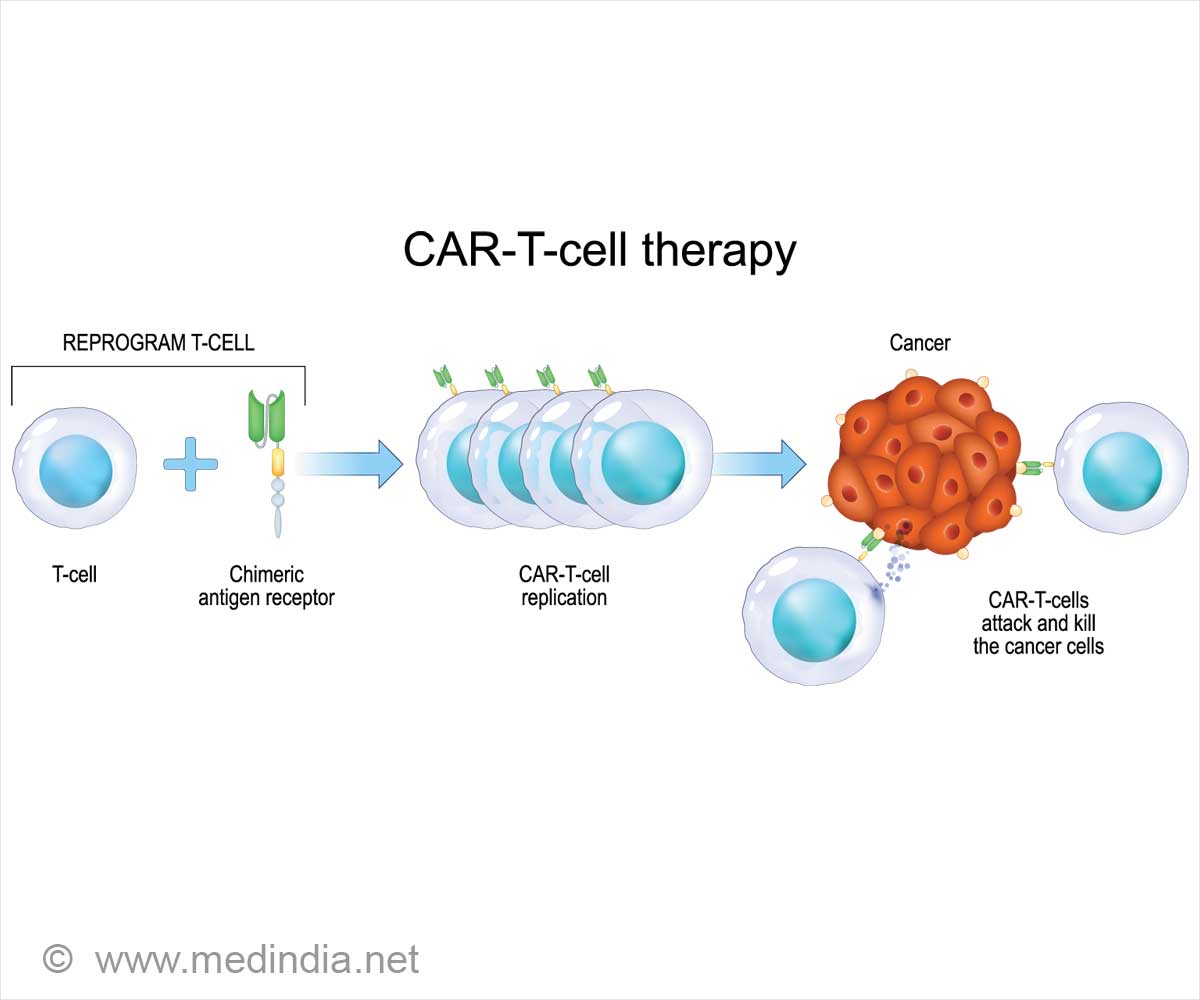
Go to source). Unlike chemotherapy or immunotherapy, which rely on commonly produced drugs, CAR T-cell therapy utilizes a patient’s cells. In this process, T-cells are modified in the lab to enhance their ability to attack tumors.
The engineered cells are infused back into the patient’s bloodstream after being conditioned to multiply effectively. Currently, CAR T-cell therapy is approved for treating leukemias and lymphomas.
Patients receiving CAR T-cell treatment for multiple myeloma or B-cell lymphoma are specifically warned in the boxed warnings about the risk of acquiring new T-cell malignancies unrelated to their original conditions.
Data from the FDA Adverse Event Reporting System served as a major basis for the decision. However, other researchers are concerned that the data may contain inborn flaws, such as reporting bias.
CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy) involves modifying a patients T-cells to better target and destroy cancer cells. #cellfreedna #tcelltherapy #medindia’
Key Factors in Assessing SPM Risk
When evaluating the risk of secondary primary malignancies (SPMs), several variables were considered, including:- Age
- Length of follow-up
- Type of CAR T-cell therapy received
- Initial diagnosis
- Prior treatments
Approved CAR T-Cell Therapies
Researchers conducted a systematic review and meta-analysis of clinical studies to determine which of the six CAR T-cell therapies are currently approved for adult patients with lymphoma or multiple myeloma.Examples of CAR T-cell therapies currently approved include:
- Tisagenlecleucel, also known as tisa-cel (Kymriah)
- Axicabtagene ciloleucel, also known as axi-cel (Yescarta)
- Brexucabtagene autoleucel, also known as brexu-cel (Tecartus)
- Lisocabtagene maraleucel, also known as liso-cel (Breyanzi)
- Idecabtagene vicleucel, also known as ide-cel (Abecma)
- Ciltacabtegene autoleucel, also known as cilta-cel (Carvykti)
To meet the inclusion criteria, the studies needed to include the entire follow-up time, which ranged from 6.6 months to 65.4 months. A total of 326 SPMs from 5,517 patients were included in the final selection, which comprised seven real-world investigations and 18 clinical trials.
The SPM rates did not significantly change across patients with different cancer types or between patients who got different CAR T-cell products.5.8% of patients experienced an SPM at a follow-up of 21.7 months.
T-Cell Malignancies as Secondary Risks in CAR T-Cell Therapy
Studies found that patients who had undergone more than three lines of therapy before CAR T-cell had a considerably increased risk of SPMs, compared to patients who had received fewer than three lines of therapy.Similarly, the rate of SPMs was 4.2% across studies with a follow-up period of less than 21.7 months, and 8.5% among studies with a follow-up time of more than 21.7 months.
Hematologic malignancies, such as acute myeloid leukemia and myelodysplastic syndrome, accounted for the biggest percentage of SPMs (37%). T-cell malignancies accounted for five of the cases of the total study population.
When examining the CAR T-Cell transgene in three of these cases, it was found that the malignant T cells tested positive, suggesting that the malignancy may have originated from CAR-edited cells.
Comparing CAR T-Cell Therapy With Standard Treatments
In four clinical trials, researchers compared CAR T-cell therapy to standard treatments in 1,253 patients. They found that 5% of patients receiving CAR T-cell therapy developed secondary cancers, compared to 4.9% of those on standard treatments. This small difference wasn't significant, meaning CAR T-cell therapy didn't show a higher risk of secondary cancers compared to standard care.The study found no increased risk of secondary primary malignancies (SPMs) with CAR T-cell therapy compared to standard treatments. The data suggest that warning labels might scare patients, despite the low risk.
Factors such as the number of prior treatments and longer follow-up times could influence SPM risk. CAR T-cell therapy, which has demonstrated survival benefits in treating resistant large B-cell lymphoma, should not be avoided due to the minimal risk of new T-cell cancers.
Further research is needed to understand the role of CAR T-cell therapy in developing SPMs and to improve individual risk assessments. Accurate long-term reporting in clinical trials is crucial. The study's limitations include variability in the data and missing information about patients' previous treatments and health conditions, which could impact SPM risk.
Reference:
- Second primary malignancies after CAR T-cell therapy: A systematic review and meta-analysis of 5,517 lymphoma and myeloma patients - (https://aacrjournals.org/clincancerres/article-abstract/doi/10.1158/1078-0432.CCR-24-1798/747919/Second-primary-malignancies-after-CAR-T-cell)
Source-Medindia