Treating a common heart rhythm disorder by burning heart tissue with a catheter works dramatically better than drug treatments, a major international study has found.
A major international study when it comes to treating a common heart rhythm disorder, burning heart tissue with a catheter is much more effective than drug treatments.
One year after undergoing a treatment called catheter ablation, 63 percent of patients with an irregular heartbeat called atrial fibrillation were free of any recurrent atrial arrhythmias or symptoms. By comparison, only 17 percent of those treated with drugs were arrhythmia-free. Results were so convincing the trial was halted early.The ablation group also scored significantly higher on a quality-of-life scale.
The study included 167 patients at 19 centers, including 15 centers in the United States. Lead researcher Dr. David Wilber presented results at Heart Rhythm 2009, the Society's 30th Annual Scientific Sessions. Wilber is director of the Cardiovascular Institute at Loyola University Stritch School of Medicine in Maywood, Il.
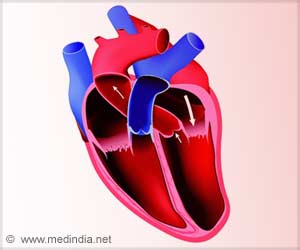
Atrial fibrillation, often called A-Fib, is the most common form of irregular heartbeat. Electrical signals, which regulate the heartbeat, become erratic. Instead of beating regularly, the upper chambers of the heart quiver. Not all the blood gets pumped out, so clots can form. Atrial fibrillation can lead to strokes and heart failure.
A-Fib patient Robin Drabant, 35, of Hanover Park, Il., said the condition once "made me feel like I was 90 years old with a failing heart." She was on a maximum dose of an A-Fib medication, which caused fatigue. Despite the drug, she still had episodes almost every day, lasting from 10 seconds to an hour or longer. "I would lose my breath and could feel my heart racing and fluttering," she said.
Wilber performed a catheter ablation on Drabant in May, 2008, and she no longer has A-Fib episodes. "I had great results," she said.
Advertisement
More than 2 million Americans have atrial fibrillation, and there are about 160,000 new cases each year. The number is increasing, due in part to the aging population and the obesity epidemic.
Advertisement
In the ablation procedure, an electrophysiologist destroys small areas of heart tissue that are responsible for the erratic electrical signals. A catheter (thin flexible tube) is guided through blood vessels to the heart. The tip of the catheter delivers radiofrequency energy that heats and destroys tissue. Possible adverse effects include irritation of the lining of the heart, fluid in the lungs or around the heart, bleeding, clots and stroke.
In the study, 106 patients with frequent episodes of atrial fibrillation were randomly assigned to undergo ablation and 61 similar patients were randomly assigned to receive drug therapy. All patients had experienced at least three episodes of atrial fibrillation during the previous six months and had failed at least one attempt to control the rhythm with drugs.
The study was funded by Biosense Webster, which makes the ThermoCool catheter used in the trial. Wilber is a consultant to the company.
The study was the largest to date to compare ablation to drug therapy for atrial fibrillation. Earlier studies involved single centers and smaller sample sizes, Wilber said. An additional study called CABANA is designed to determine whether ablation patients live longer than patients receiving medication. Researchers will follow about 3,000 patients for three years.
Source-Eurekalert