A female patient lost her vision due to P. aeruginosa (drug-resistant bacterial) infection from tainted eye drops months before the CDC’s warning in February 2023.

Outbreak and Patient Notifications
Go to source).
‘The CDC advised against using some artificial tear eye drops that were contaminated with the drug-resistant gram-negative bacterium Pseudomonas aeruginosa.’





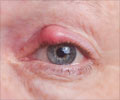
Eventually, infectious disease physicians and microbiologists identified her contaminated eye drops as the source of the infection. P. aeruginosa is a pathogenic, gram-negative bacterium that’s resistant to treatment with most antibiotics.It can cause swimmer’s ear — a painful infection of the outer ear canal — and more serious conditions, especially in people with compromised immune systems. But the case in Cleveland was unusual.
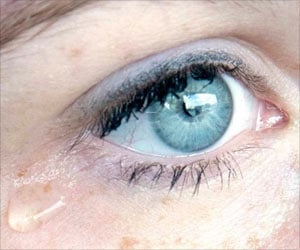
The patient was initially reported to an outpatient eye clinic with blurry vision. From there, she was sent to the emergency department at the hospital, where she was evaluated by ophthalmologists.
They cultured the infection, prescribed a combination of strong antibiotic eye drops, and sent her home. But the next day, the eye was worse—and she visited a cornea specialist. The patient had noticed a yellow discharge on her pillow, and she had not been swimming. At that point, she was referred to microbiologists and infectious disease experts at the hospital (2✔ ✔Trusted Source
A Dangerous Eye Infection From Tainted Eye Drops, Months Before the CDC’s Warning
Go to source).
A sample from the patient was sent to the lab at Case Western Reserve University School of Medicine. Then, scientists identified a P. aeruginosa isolate that matched the genetic material found in the EzriCare artificial tear eye drops she had been using. Then researchers connected the eye infection—and the ulcer it had caused—to the contaminated drops.
Advertisement
Since issuing the warning in February, the CDC has identified infectious cases due to P. aeruginosa as early as spring 2022. Although the contaminated product has been pulled from stores and can no longer be purchased, it may still pose a risk.
- Outbreak of Extensively Drug-resistant Pseudomonas aeruginosa Associated with Artificial Tears - (https://www.cdc.gov/hai/outbreaks/crpa-artificial-tears.html)
- A Dangerous Eye Infection From Tainted Eye Drops, Months Before the CDC’s Warning - (https://asm.org/Press-Releases/2023/May/A-dangerous-eye-infection-from-tainted-eye-drops,)
Source-Eurekalert