While a landmark study by the Southwest Oncology Group (SWOG) claimed that chemotherapy combined with tamoxifen could be beneficial for postmenopausal breast cancer patients.
While a landmark study by the Southwest Oncology Group (SWOG) claimed that chemotherapy combined with tamoxifen could be beneficial for postmenopausal breast cancer patients, another study has claimed that this may not be true for everyone.
The second study found that a multigene test on tumors could identify a subset of patients who may not benefit from that chemotherapy.Both studies, led by Dr. Kathy Albain, of Loyola University Health System, are based on a randomized phase III clinical trial of 1,477 postmenopausal women.
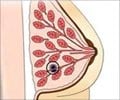
All of the women had estrogen receptor-positive (ER+) breast cancer that had spread to the lymph nodes under the arm, known as the axillary lymph nodes.
All women in the trial got daily tamoxifen for up to five years, long the standard therapy for treating ER+ breast cancer, the most common form of the disease.
Estrogen latches on to receptors on these cancer cells and promotes tumor growth. Tamoxifen blocks those receptors, locking estrogen out.
Of the 1,477 women in the trial, 361 got only tamoxifen. The rest got tamoxifen plus a regimen of a three-drug chemotherapy treatment known as CAF (cyclophosphamide, Adriamycin, and 5-fluorouracil).
Advertisement
The team also found that those who got the chemotherapy before the tamoxifen did better than those who got both simultaneously.
Advertisement
"Ten years after the start of their treatment, 68 percent of women who received chemotherapy followed by tamoxifen were still alive, while only 60pc of women in the tamoxifen-only arm lived for at least ten years," said Dr. William Barlow, who served as lead statistician on both studies.
In the second study, researchers wanted to determine if subsets of women in the SWOG trial could be identified who did not benefit from chemotherapy despite being at higher risk due to lymph node involvement.
They analysed tumour specimens from the trial using a genetic test called the 21-gene recurrence score assay (Oncotype DX).
The assay measures the expression or activity level of 21 specific genes within a tumour sample, and the score predicts risk of recurrence.
The study found that women whose tumors scored low on the genetic test appeared to get little or no benefit from CAF chemotherapy added to tamoxifen, while those with higher scores seemed to derive major benefit from this addition.
The 21-gene recurrence score test is now routinely used on the tumors of many patients whose breast cancer has not spread to their lymph nodes - node-negative breast cancer.
Doctors use the assay score to help them decide whether a patient is likely to benefit from a course of chemotherapy.
The researchers conclude that this test may similarly predict chemotherapy benefit in patients whose breast cancer has spread to their lymph nodes.
The first study has been published in the journal the Lancet, the second in the Lancet Oncology.
Source-ANI
SRM