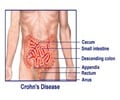
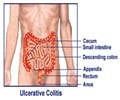
A new study reveals that the type of IBD cases being used to conduct major randomized controlled trials for development of new therapies are not the typical cases.
"Many outpatients with moderate-to-severe inflammatory bowel disease may not qualify for trials of new treatment options," said Christina Ha, MD, of the Johns Hopkins School of Medicine and lead author of this study. "Because of the heterogeneity of the inflammatory bowel disease patient population in terms of age of onset, disease behavior and severity, many patients may not meet the strict entry criteria for clinical trial participation. However, we often presume similar therapeutic effect among our clinic patients as demonstrated in clinical trials."
Researchers from the Johns Hopkins School of Medicine, Mount Sinai School of Medicine and Dartmouth-Hitchcock Medical Center performed a retrospective cohort study of 206 adult patients with moderate-to-severe IBD who went to the Mount Sinai Medical Center for an adjustment of their medical therapy from January 2008 to June 2009. Of these patients, only 31.1 percent would have been eligible to participate in a randomized controlled clinical trial of biologics. Reasons for exclusion included structuring or penetrating Crohn's disease, high-dose intake of steroids, comorbidities or prior exposure to biologics, or treatment with topical therapies.
Results from randomized clinical trials form the basis for FDA approval of treatments, expert recommendations and guidelines for the management of IBD patients, and are currently the best approach available to study treatment effects. However, by limiting the available pool of potential study patients through strict inclusion and exclusion criteria in an effort to maximize clinical trial results, these trial results may have limited applicability in the real-world doctor's office.
In addition, limiting selection criteria requires larger study populations, longer durations of follow-up observation and substantially greater cost. However, shifting to a more pragmatic-based approach with minimal study exclusions may help physicians generalize study results to their day-to-day practice as well as support randomized controlled trial findings in a more common clinical practice setting.
Advertisement