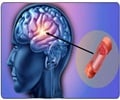
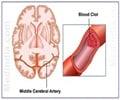
According to a trial a stroke survivor's chances of living independently after 90 days are not improved by the use of devices inserted into the artery to dissolve a stroke-causing clot.
"This is the largest trial of an intra-arterial intervention for acute ischemic stroke, and is important because it shows that the addition of devices to treat clots within the brain artery was no more effective than treatment with IV t-PA alone," said Walter J. Koroshetz, M.D., deputy director, NINDS. "Such devices are already widely used in clinical practice based on dramatic improvements seen in individuals, but without evidence supporting their overall clinical effectiveness in a large group."
Intra-arterial treatment is known to be associated with significant risk; 16 percent of the patients in the study had complications from the minimally invasive surgical procedure. The Interventional Management of Stroke, or IMS-III, is the only trial designed to assess the overall balance between risk and benefit of intra-arterial treatment in acute stroke patients.
Eliminating a clot from within a vessel involves threading a catheter through an artery in the groin up to the site of a clot in the brain, and then administering t-PA directly on the clot, which dissolves it, or removing it via coiled wires or a stent system. Based on earlier studies, researchers anticipated that adding an intra-arterial approach to standard IV t-PA would reduce stroke-related disability by providing a means to eliminate very large clots that would otherwise persist despite IV t-PA, according to the researchers led by Joseph Broderick, M.D., of the University of Cincinnati Academic Health Center.
Participants, ages 18-82 years, were enrolled in the study starting in 2006. By April 2012, after 656 of the 900 planned participants had been treated, the study's Data and Safety Monitoring Board advised that the investigation should be stopped early because 90-day outcomes showed that the benefit was not substantially different between the groups. Patients in the IV t-PA alone group (39 percent) were just as likely as those in the combination therapy group (41 percent) to be functionally independent.
All patients in the study received IV t-PA therapy within three hours of their stroke symptoms. Within 40 minutes of that therapy, 434 patients who were randomized to the device-treatment group underwent angiography to identify arterial blockages. Those patients found to have a treatable blockage received intra-arterial therapy with one of five types of devices. The remaining 222 patients were randomized to receive IV t-PA alone.
Advertisement
The time to intra-arterial treatment was 32 minutes longer in IMS III compared with the two pilot studies that preceded it. This may be an important reason for the lack of clinical benefit despite substantially better revascularization with intra-arterial therapy as compared to IV t-PA. "We probably did not open the arteries quickly enough," said Dr. Broderick.
Advertisement
IMS III sheds light on research gaps that need to be addressed in trials to develop evidence-based recommendations on the use of intra-arterial devices, said Scott Janis, Ph.D., program director, NINDS.
"These devices still may play a critical role in improving outcomes among people with serious strokes involving clots that cannot be cleared or are cleared very slowly by standard IV t-PA" Dr. Janis said.
"Future research may show that newer generations of Food and Drug Administration-cleared devices and improved patient care may change the balance of the risk/benefit ratio of intra-arterial treatment for acute stroke. In addition, neuroimaging might be useful in selecting patients who can benefit from intra-arterial therapy," said Dr. Koroshetz.
Additional information from subset analyses of the IMS III data will be presented at the International Stroke Conference in Honolulu, February 6-8, 2013.
Source-Eurekalert