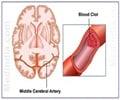
Merck & Co.'s experimental anti-platelet drug vorapaxar reduces the risk of heart attacks but increases the risk of significant bleeding.

From January 2011 a safety committee overseeing the 26,449-patient study had questioned its efficacy as they found that the new type of anti-platelet drug was not appropriate for patients who had suffered a stroke, due to bleeding risk.
Source-Medindia