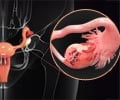
A recent clinical trial investigating the effectiveness of a combination of immunotherapy treatments could be more effective for ovarian cancer patients.
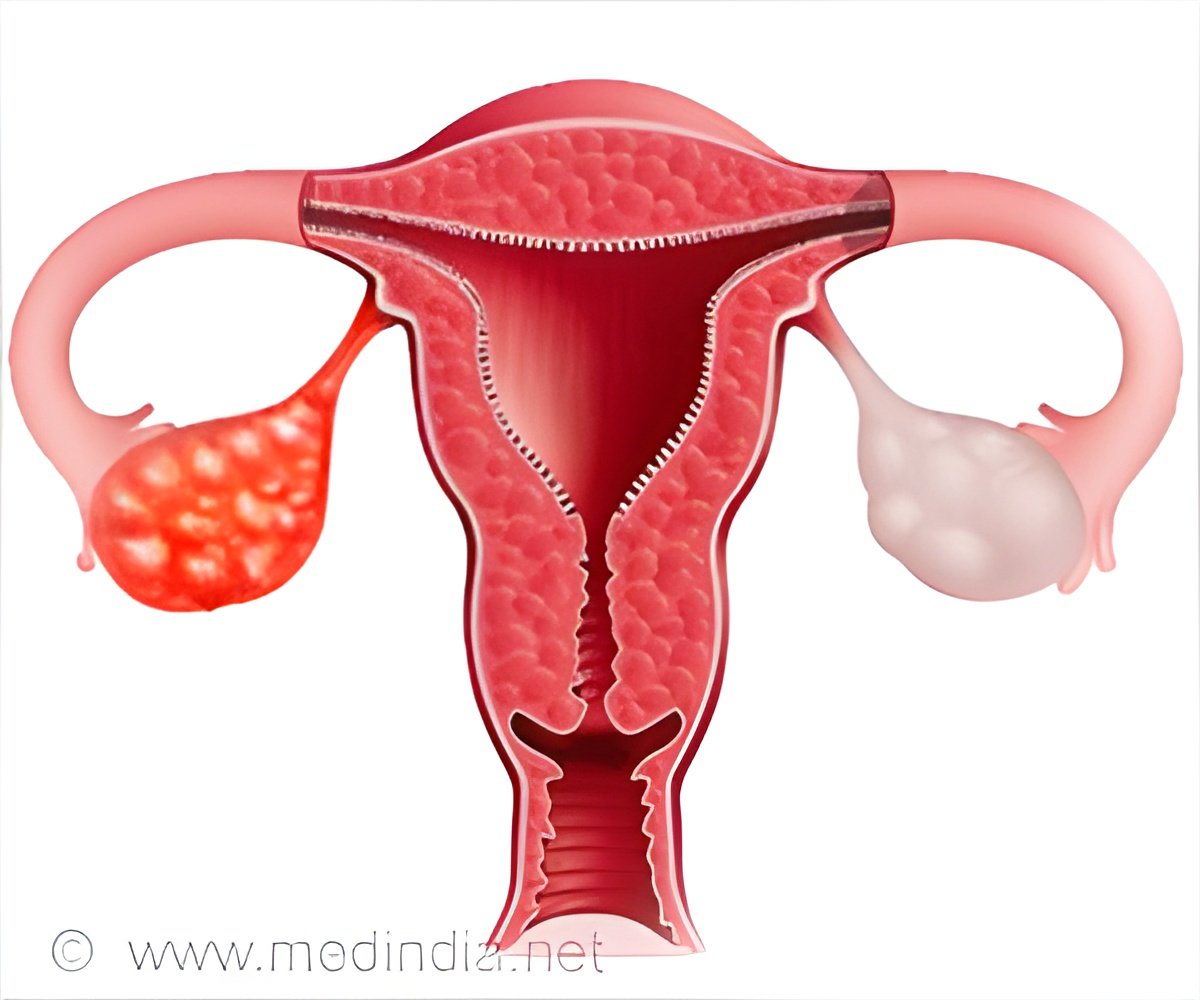
‘A majority of patients’ cancers will eventually develop resistance to platinum-based chemotherapy. For platinum-resistant ovarian cancers, the standard of care is a non-platinum chemotherapy, but this treatment offers a low likelihood of long-term effectiveness.’





Herzog explained that ovarian cancer could be a difficult disease to recognize and diagnose at first due to vague and mild symptoms, making it difficult to treat. Treatments include surgery and platinum-based chemotherapies, which contain the chemical element platinum as part of their molecular make-up and form structures that prevent cancer cells from growing or causing them to die.
Ovarian Cancer Trial Design
The trial will examine the effectiveness of combining two immunotherapy drugs called nemvaluekin alfa and pembrolizumab compared to the standard of non-platinum-based chemotherapy. Immunotherapy helps boost and train the body’s own immune system to identify and kill diseased cells.“There is a high unmet clinical need for treatment of patients with platinum-resistant ovarian cancer, and the ARTISTRY-7 trial provides a pathway to meet those unmet needs by incorporating cutting edge scientific advancements in targeted cancer therapy in a treatment regimen in combination with pembrolizumab,” said Herzog, deputy director of the University of Cincinnati Cancer Center, clinical director and Paul and Carolyn Flory Professor in Gynecologic Oncology in the UC College of Medicine and a UC Health gynecologic oncologist.
Pembrolizumab, sold under the brand name Keytruda, is a well-established immunotherapy drug used to treat a variety of cancers. Nemvaleukin alfa is a novel drug created by drug company Alkermes that received Food and Drug Administration fast track designation last fall to accelerate the review of the drug’s safety and effectiveness.
“Nemvaluekin alfa is a drug that works with your body’s immune system to expand the cancer-fighting immune cell,” Herzog said. “It is based on an established immune pathway which has shown promise in certain cancer types.”
Herzog said the trial would enroll and randomly assign 376 patients into one of four study arms. One group of patients will receive both immunotherapy drugs, one group will receive nemvaluekin alfa only, another group will receive pembrolizumab only and the final group will receive standard chemotherapy treatments.
Advertisement
ARTISTRY-7 is a Phase 3 trial. It is designed to confirm preliminary evidence from previous trials that the treatment option is safe, beneficial, and effective for the patient population, and Herzog said the preliminary evidence has been promising. Alkermes is the trial sponsor, and Herzog helped design the study for the specific patient population it will target.
Advertisement
“If this trial is successful, patients will have a novel treatment option where limited options exist, and it allows a break from repetitive chemotherapy, thereby potentially having better efficacy and safety profiles,” he said.
Source-Eurekalert