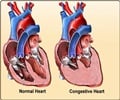
Personalized care for congestive heart failure, also known as ischemic cardiomyopathy can now be possible with a new study that describes the underlying mechanism that reprograms the hearts of patients with ischemic cardiomyopathy
‘Personalized care for congestive heart failure, also known as ischemic cardiomyopathy can now be possible with a new study that describes the underlying mechanism that reprograms the hearts of patients with ischemic cardiomyopathy’





The study used heart tissue samples collected at UAB during surgeries to implant small mechanical pumps alongside the hearts of patients with end-stage heart failure that assist in the pumping of blood. As a routine part of this procedure, a small piece of heart tissue is excised and ultimately discarded as medical waste. The current study acquired these samples from the left ventricles of five ischemic cardiomyopathy patients and six non-ischemic cardiomyopathy patients, all men between ages 49 and 70. The research team, led by Adam Wende, Ph.D., assistant professor in the UAB Department of Pathology, found that epigenetic changes in ischemic cardiomyopathy hearts likely reprogram the heart's metabolism and alter cellular remodeling in the heart. Epigenetics is a field that describes molecular modifications known to alter the activity of genes without changing their DNA sequence.
One well-established epigenetic change is the addition or removal of methyl groups to the cytosine bases of DNA. Generally, hyper-methylation is associated with reduction of gene expression, and conversely, hypo-methylation correlates with increased gene expression.
Wende and colleagues found an epigenetic signature in the heart of patients with ischemic cardiomyopathy that differed from the non-ischemic hearts. Furthermore, this signature was found to reflect a long-known metabolic change in ischemic cardiomyopathy, where the heart's preference of metabolic fuel switches from using oxygen to produce energy in cells, as healthy hearts do, to an anaerobic metabolism that does not need oxygen. This anaerobic metabolic preference is seen in fetal hearts; however, after birth, the baby's heart quickly changes to oxidative metabolism.
"Altogether, we believe that epigenetic changes encode a so-called 'metabolic plasticity' in failing hearts, the reversal of which may repair the ischemic and failing heart," Wende said.
Advertisement
This contribution by EZH2 offers a new molecular target for further mechanistic studies that may aid precision-based heart disease therapies. Of note, co-author Sooryanarayana Varambally, who has spent over 15 years studying this protein, has already made progress using small-molecular inhibitors to regulate EZH2 to treat various cancers.
Advertisement
Pepin is a sixth-year M.D.-Ph.D. student at UAB and is currently completing the Ph.D. portion of his training in the Medical Scientist Training Program.
The UAB team also performed cell culture experiments showing repression of KLF15 after EZH2 over-expression in rat cardiomyoblasts, and they demonstrated that EZH2 over-expression depended on EZH2's having an intact SET catalytic domain.
Source-Eurekalert