The U.S. Food and Drug Administration updated the warning label on the Ortho Evra birth-control patch, cautioning users about the higher risk of blood clots from it.
The U.S. Food and Drug Administration on Wednesday reported having updated the warning label on the Ortho Evra birth-control patch, cautioning users about the higher risk of blood clots from it.
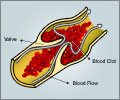
The drug manufacturer Johnson and Johnson’s commissioned study revealed that drug users faced two times the risk of clots in the legs and lungs compared to women taking traditional birth-control pills.Another study commissioned by Johnson and Johnson, however, found no difference in risk between the patch and the pill. The FDA said, "Even though the results of the two studies are conflicting, the results of the second epidemiology study support FDA's concerns regarding the potential for Ortho Evra use to increase the risk of blood clots in some women."
Dr. Daniel Shames, the acting deputy director of FDA's Office of Drug Evaluation in the Center for Drug Evaluation and Research, said, "Blood clots occurring in the legs or lungs are serious and rare events that are a potential risk for all hormonal contraceptive therapies."
Shames said that risk factors for blood clots associated with contraceptives include smoking, inactivity, obesity, surgery and high doses.
The FDA has also required Johnson and Johnson to conduct a longer follow-up study of women using the patch to check for developments of heart attack, blood clots and stroke. Currently, the FDA believes the patch is safe for most women. Shames said, "Ortho Evra risk profile is acceptable for a highly effective contraceptive."
However the FDA’s concerns about the potential risk of blood clots in some women could not be ruled.
Advertisement
According to the label change women using the weekly patch which is the only birth-control patch approved by the FDA is taking in substantially more estrogen than women who take a daily birth-control pill. These pills typically contain 35 micrograms of estrogen. These higher levels of estrogen put some women at increased risk of blood clots.
Advertisement
He added, "My advice is that for a patient who had been on the patch for any period of time and is doing well, I don't see a need to take her off. With most of the estrogen-related blood clots, the risk is greatest in the first six months of use."
Source-Medindia
NLA