Activation of the chloride channel in the lungs could help in the functioning of the lungs in those with cystic fibrosis.
‘TMEM16 scramblase, which functions as a lipid transporter and plays an important role in blood coagulation can activate calcium and help in the treatment of cystic fibrosis.’





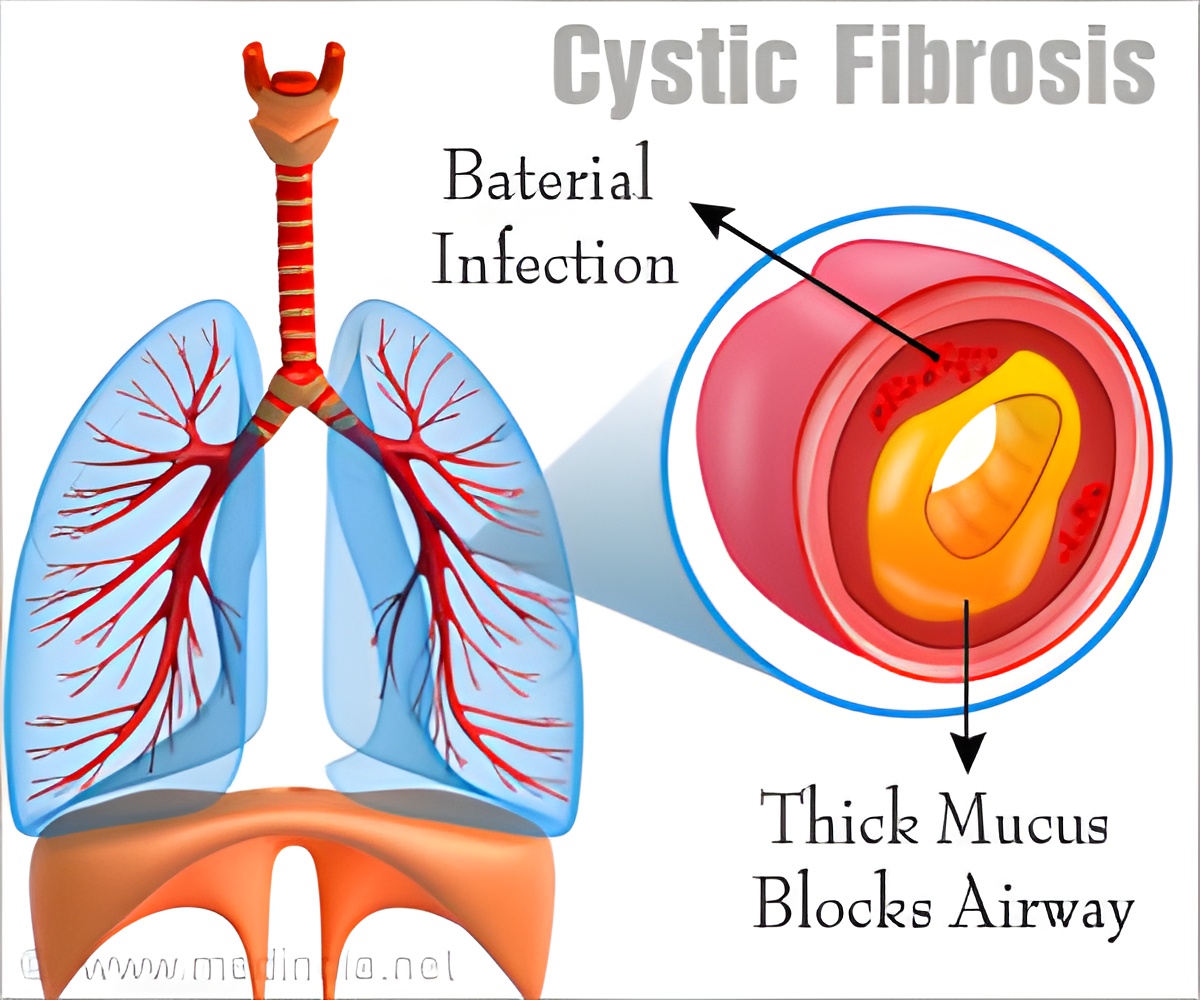
Since TMEM16A is expressed in the same epithelium as CFTR, its activation could restore hydration of the mucus layer. TMEM16A is part of a protein family whose members facilitate the flow of negatively charged chloride ions or lipids across the cell membrane. Structure of a Chloride Channel Determined
The structure of a TMEM16 scramblase, which functions as a lipid transporter and plays an important role in blood coagulation, was already known from previous work. Researchers of the Department of Biochemistry at the University of Zurich have now also succeeded in decrypting the structure of the chloride channel TMEM16A.
To do so, the team led by Professor Raimund Dutzler used cryo-electron microscopy (cryo-EM), a technique whose pioneers were recently awarded the Nobel Prize in Chemistry. "The molecular architecture of this membrane protein is crucial for the targeted development of drugs for treating cystic fibrosis," emphasizes Dutzler.
Discovery of a novel activation mechanism
Advertisement
While its general architecture resembles scramblases of the same family, there are distinct differences in the pore region located in each subunit of the dimeric protein. Scramblases contain a membrane-exposed polar furrow, which allows for the diffusion of lipid headgroups across the lipid bilayer. In contrast, at the same location, TMEM16A forms an hourglass-shaped protein-enclosed channel, which is closed in the absence of calcium.
Advertisement
"This activation mechanism is unique since the bound calcium ions directly change the structure and electrostatics of the ion permeation pore," explains Cristina Paulino, lead author of the study.
Paving the way for novel therapies
The findings that describe the structure and function of TMEM16A pave the way for a mechanistic understanding of this important family of membrane proteins, and they provide a promising template for developing drugs for the treatment of cystic fibrosis.
"Substances leading to the activation of the TMEM16A would compensate the defect in the secretion of chloride ions in the lung," states Raimund Dutzler.
Source-Eurekalert