The US Food and Drug Administration (FDA) has ordered hormone drugs for prostate cancer carry warnings of raised risk of diabetes and heart problems, including death.
The US Food and Drug Administration (FDA) has ordered hormone drugs for prostate cancer carry warnings of raised risk of diabetes and heart problems, including death. The revised labels will include cautions in the warnings and precautions section about these potential risks.
The medications include Abbott Laboratories Inc's (ABT.N) Lupron, AstraZeneca Plc's (AZN.L) Zoladex and Sanofi-Aventis SA's (SASY.PA) Eligard.The drugs, known as gonadotropin-releasing hormone (GnRH) agonists, are used to suppress the production of testosterone, a hormone that helps fuel prostate cancer growth. The drugs are approved to relieve symptoms of advanced prostate cancer in a treatment known as androgen deprivation therapy.
The FDA said the risk of diabetes and heart disease in men treated with the drugs appeared to be low, but that patients should be regularly monitored for increased blood sugar or possible signs of heart damage.
Doctors also should evaluate a patient's risk for diabetes and heart disease before starting treatment and weigh potential side effects versus benefits, the FDA said.
Other GnRH drugs include Watson Pharmaceuticals Inc's (WPI.N) Trelstar, Endo Pharmaceuticals Holdings Inc's (ENDP.O) Vantas, and Pfizer Inc's (PFE.N) Synarel. Several generic versions also are sold.
The FDA's notification to manufacturers about the GnRH agonists is based on the agency's review of several published studies, according to the agency.
Advertisement
"There is no convincing evidence that hormone blockade shortens life or causes excess heart attacks if weight gain is attended to and blood sugar levels are kept in check," said Mark Scholz, MD, from the Prostate Cancer Research Institute in Los Angeles, California.
Advertisement
The use of androgen deprivation therapy has been controversial in other ways as well. The approach has been "overused," according to a pair of experts in separate editorials in two major medical journals in 2009.
Professional societies have been concerned about adverse events related to androgen deprivation. In February of this year, an advisory jointly issued by the American Heart Association, the American Cancer Society, and the American Urological Association said that "there may be a relationship" between androgen deprivation therapy for prostate cancer and cardiovascular risk.
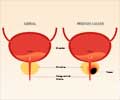
Prostate cancer is the second most common cancer among U.S. men. More than 217,000 new cases are expected to be diagnosed in the United States this year and about 32,000 men will die from the disease, according to government estimates.
Source-Medindia