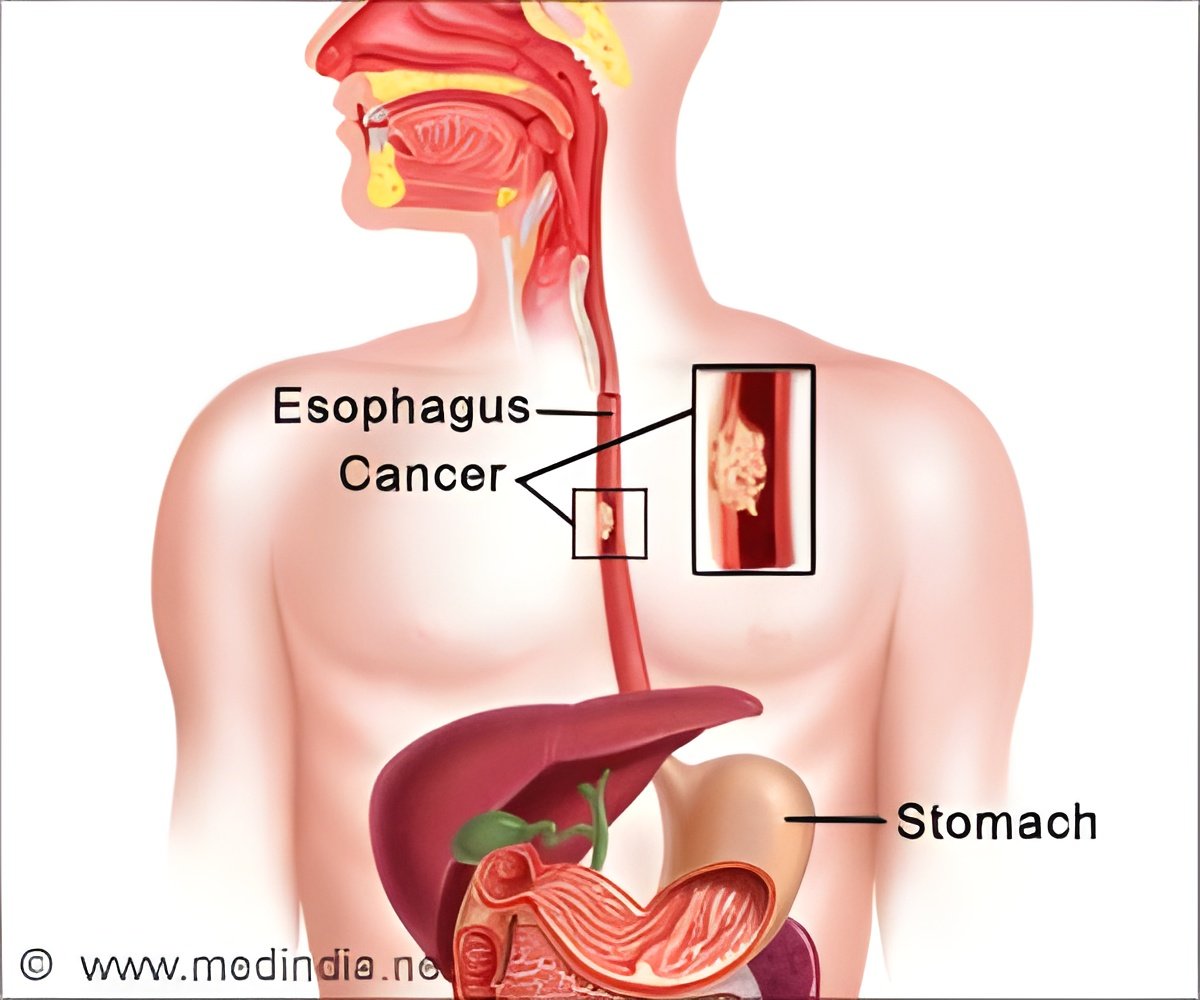
More than 2,000 genetic mutations in tissue samples of esophageal tumors have been identified by researchers.
‘Deciphering the genomic diversity and evolution of tumors can provide a basis for identifying new targets and designing personalized medicine strategies.’





The findings
reveal that even different areas of individual tumors have various
genetic patterns. The study results, published in the journal Nature Genetics, help
explain why it is so difficult to battle cancer by targeting a specific
genetic defect. A surgeon who performs a single biopsy on a patient's tumor can decode only part of the tumor and its genetic variations. Additionally, cancer cells constantly change their makeup. "A tumor is not a single disease," said Dechen Lin, assistant professor and research scientist in the Division of Hematology and Oncology in the Cedars-Sinai Department of Medicine. "It's many diseases within the same person and over time. There are millions of cells in a tumor, and a significant proportion of them are different from each other." Lin was the project coordinator for the multicenter study.
To create their catalog of mutations, the study's investigators called on high-powered computers to compile genetic data on 51 tumor samples taken from 13 patients. Through complex algorithms, they analyzed both the genes and the processes, known as epigenetics, that turned the genes' activities on and off within the cancer cells.
Using these techniques, the investigators identified 2,178 genetic variations in the sampled tumors. Dozens of the variations involved genes known to be associated with enabling the development of cancer. The most striking finding was that many important mutations were detected only in some areas of a tumor, highlighting the complexity of the cancer cells. This finding also demonstrated the potential for inaccurate interpretation of a cancer's genetic makeup using the single-biopsy method, which is the standard approach in the clinic.
Besides cataloging these genetic variations, the study's investigators reconstructed a "biography" of the tumors, showing when some of these variations first appeared in the life cycle of the disease.
Advertisement
To meet the challenge of integrating this diverse data, Huy Dinh, a project scientist in Berman's laboratory and one of the study's co-lead authors, developed innovative computational methods.
Advertisement
"Evidence suggests that tumor heterogeneity is one of the major causes of drug resistance and treatment failure in cancer," said H. Phillip Koeffler, professor of Medicine and the Mark Goodson Chair in Oncology Research at Cedars-Sinai. "In light of this situation, deciphering the genomic diversity and evolution of tumors can provide a basis for identifying new targets and designing personalized medicine strategies." Koeffler was the other co-senior author of the study.
Source-Eurekalert