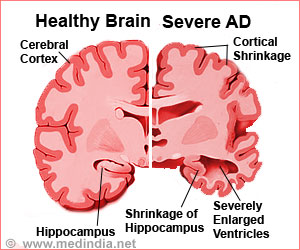
Collaboration for Alzheimer's Prevention(CAP) claims that sharing data on clinical trials among researchers will accelerate the evaluation of prevention methods.

The founding members of CAP include representatives from the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) Study, Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), Alzheimer's Prevention Initiative (API), the Food and Drug Administration (FDA), the National Institute on Aging (NIA), the Alzheimer's Association and FBRI. Researchers from the TOMMORROW Trial later joined CAP, bringing the perspective of a study that is funded by industry rather than government agencies such as the NIA.
"When the Alzheimer's Association teamed up with FBRI to convene representatives from Alzheimer's prevention trials, we wanted to tap into a spirit of collaboration among these researchers that would make a positive impact on the clinical trials process. It was apparent from early conversations that all the prevention trials, and the Alzheimer's research field, would benefit from ongoing conversation and coordination among the scientists, and with the regulatory agencies. Before CAP, researchers had one-off conversations and conducted trials in a way that created unnecessary overlap. This collaboration eliminates that among CAP participants and aligns everyone's work where it makes most sense." said Maria Carrillo, chief science officer at the Alzheimer's Association.
"FBRI, alongside the Alzheimer's Association, believes that prevention research is the future in the fight against Alzheimer's. CAP is strategically important because it brings together stakeholders in a way that yields practical benefits - in this case, accelerating the evaluation of prevention trials. The lessons learned from A4, API, DIAN-TU and TOMMORROW will also set the stage for investigators who want to test other prevention therapies,"said Stacie Weninger, executive director of FBRI and CAP's chairperson.
CAP aims for researchers to provide assistance to each other in the development of trial outcomes (cognitive and clinical endpoints, and biomarkers), standardization of sample and data collection (clinical and cognitive data, imaging, and biofluids), and recruitment and retention of study participants (registry development and risk disclosure).
Reisa Sperling, director of the Center for Alzheimer's Research and Treatment at Brigham and Women's Hospital, Harvard Medical School, added, "Early discussions with government regulators - made possible through CAP - have greatly helped move clinical trials forward. In the case of the A4 Trial, we were able to advance the trial along to the next phase of the clinical trial process with this regulatory insight."
Advertisement
"Collaboration across CAP member studies has the high potential of providing unique insight into the early stages of cognitive decline in Alzheimer's, through the very different strategies used in these trials to enrich the subject groups for AD risk," said Kathleen Welsh-Bohmer, neuropsychology lead for the TOMMORROW Study at Duke University.
Advertisement
With more than five million Americans currently living with Alzheimer's disease and that number projected to climb to 13.8 million by 2050, it is more critical than ever to find faster methods to evaluate promising preclinical Alzheimer's treatments, develop new avenues for researchers to share insights and experiences, and expedite the initiation and performance of preclinical Alzheimer's trials.
Source-Medindia