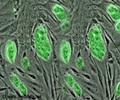
A team of scientists led by Dr. Dieter Egli has created the first disease-specific embryonic stem cell line with two sets of chromosomes, using somatic cell nuclear transfer.
The investigators overcame the final hurdle in making personalized stem cells that can be used to develop personalized cell therapies. They demonstrated the ability to make a patient-specific embryonic stem cell line that has two sets of chromosomes (a diploid state), the normal number in human cells. Reports from 2013 showed the ability to reprogram fetal fibroblasts using SCNT; however, this latest work demonstrates the first successful derivation by SCNT of diploid pluripotent stem cells from adult and neonatal somatic cells.
"From the start, the goal of this work has been to make patient-specific stem cells from an adult human subject with type 1 diabetes that can give rise to the cells lost in the disease," said Dr. Egli, the NYSCF scientist who led the research and conducted many of the experiments. "By reprograming cells to a pluripotent state and making beta cells, we are now one step closer to being able to treat diabetic patients with their own insulin-producing cells."
"I am thrilled to say we have accomplished our goal of creating patient-specific stem cells from diabetic patients using somatic cell nuclear transfer," said Susan L. Solomon, CEO and co-founder of NYSCF. "I became involved with medical research when my son was diagnosed with type 1 diabetes, and seeing today's results gives me hope that we will one day have a cure for this debilitating disease. The NYSCF laboratory is one of the few places in the world that pursues all types of stem cell research. Even though many people questioned the necessity of continuing our SCNT work, we felt it was critical to advance all types of stem-cell research in pursuit of cures. We don't have a favorite cell type, and we don't yet know what kind of cell is going to be best for putting back into patients to treat their disease."
The research is the culmination of an effort begun in 2006 to make patient-specific embryonic stem cell lines from patients with type 1 diabetes. Ms. Solomon opened NYSCF's privately funded laboratory on March 1, 2006, to facilitate the creation of type 1 diabetes patient-specific embryonic stem cells using SCNT. Initially, the stem cell experiments were done at Harvard and the skin biopsies from type 1 diabetic patients at Columbia; however, isolation of the cell nuclei from these skin biopsies could not be conducted in the federally funded laboratories at Columbia, necessitating a safe-haven laboratory to complete the research. NYSCF initially established its lab, now the largest independent stem cell laboratory in the nation, to serve as the site for this research.
In 2008, all of the research was moved to the NYSCF laboratory when the Harvard scientists determined they could no longer move forward, as restrictions in Massachusetts prevented their obtaining oocytes. Dr. Egli left Harvard University and joined NYSCF; at the same time, NYSCF forged a collaboration with Dr. Sauer who designed a unique egg-donor program that allowed the scientists to obtain oocytes for the research.
Advertisement
Patients with type 1 diabetes lack insulin-producing beta cells, resulting in insulin deficiency and high blood-sugar levels. Therefore, producing beta cells from stem cells for transplantation holds promise as a treatment and potential cure for type 1 diabetes. Because the stem cells are made using a patient's own skin cells, the beta cells for replacement therapy would be autologous, or from the patient, matching the patient's DNA.
Advertisement
The technique described in the report published today can also be translated for use in the development of personalized autologous cell therapies for many other diseases and conditions including Parkinson's disease, macular degeneration, multiple sclerosis, and liver diseases and for replacing or repairing damaged bones.
As part of the work, the scientists systematically analyzed the factors that affect stem-cell derivation after SCNT. The reprogramming of skin cells from a type 1 diabetes patient by SCNT has long been sought, but has been challenging to achieve because of logistical difficulties in obtaining human oocytes for research, as well as an incomplete understanding of the biology of human oocytes.
The scientists found that the addition of specific chemicals, called histone deacetylase inhibitors, and an efficient protocol for human oocyte activation were critical to achieving development to the stage at which embryonic stem cells are derived. These findings are consistent with the 2013 report by Tachibana and colleagues that used fetal cells. Though the authors of the 2013 paper also performed studies with cells of an infant with Leigh syndrome, they did not demonstrate that diploid pluripotent stem cells could be derived from these cells. Because fetal cells are less mature than the cells after birth, it was critical to determine if diploid pluripotent stem cells could be derived from the cells of both infants and adults.
As an additional optimization of the SCNT protocol, the scientists found that it was important to maintain the integrity of the plasma membrane during manipulation, and that to do so, the agent used in the manipulations had to be at a low dose. The scientists applied this optimized protocol to skin cells of a male newborn and the cells of the adult patient with type 1 diabetes. From these two cell lines, the scientists produced a total of four SCNT-derived embryonic stem cell lines. All cell lines were diploid and could give rise to neurons, pancreatic cells, and cartilage, as well as various other cell types, demonstrating their pluripotency. Importantly, the cells of the type 1 diabetes patient also gave rise to insulin-producing beta cells.
Therefore, this is the first report of the derivation of diploid pluripotent stem cells from a patient. And together with a paper published this month in Cell Stem Cell by Chung et al., it is also the first report of diploid embryonic stem cell lines derived from a human after birth.
Dr. Nissim Benvenisty and his laboratory at Hebrew University of Jerusalem collaborated on this report by demonstrating that the cells produced were, in fact, embryonic stem cells by using microarrays to perform gene expression analysis of the cells.
Dr. Rudolph Leibel, a co-author and co-director with Dr. Robin Goland of the Naomi Berrie Diabetes Center, where aspects of these studies were conducted, said, "This accomplishment is the product of an ongoing inter-institutional collaboration across scientific and clinical disciplines, supported by thoughtful philanthropy. The resulting technical and scientific insights bring closer the promise of cell replacement for a wide range of human disease."
NYSCF continues pursuing SCNT research despite many scientific obstacles and in light of the advent of induced pluripotent stem (iPS) cells, as it is not yet clear which type of stem cells will prove best for personalized treatments. Many thought that iPS cells, first created from human cells in 2007, would replace the need for patient-specific embryonic stem cells because they allow patient- and disease-specific stem cell lines to be generated by genetically reprogramming adult cells into becoming pluripotent cells. However, it is not clear how similar iPS cells are to naturally occurring embryonic stem cells, which remain the gold standard, and what will be the preferred cell type for therapies.
Though it is now possible to derive stem cell lines with a patient's genotype using iPS technology, the generation of stem cells using oocytes may have an advantage for use in cell replacement for diseases such as type 1 diabetes. The generation of pluripotent stem cell lines by SCNT uses human oocytes, while iPS cells use recombinant DNA, RNA, or chemicals, each of which requires its own safety testing and approval for clinical use. Human oocytes are already used routinely around the world to generate clinically relevant cells. The generation of pluripotent stem cell lines using human oocytes may therefore be particularly suitable for the development of cell-replacement therapies. Therefore, this work brings the scientists a significant step closer to this goal.
Drs. Mitsutoshi Yamada and Bjarki Johannesson, postdoctoral fellows at the NYSCF Research Institute, were the co-first authors of the paper.
Source-Eurekalert