The first-of-its-kind drug eluting ABSORB Bioresorbable Vascular Scaffold (BVS) has been deployed for the first time after FDA approval for commercial use.

‘The first-of-its-kind drug eluting ABSORB Bioresorbable Vascular Scaffold (BVS) has been deployed for the first time after FDA approval for commercial use.’





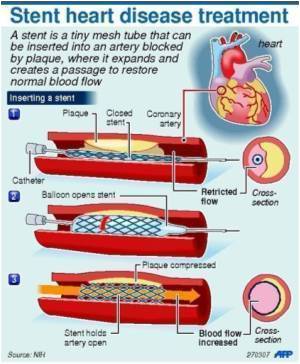
The same PinnacleHealth team that participated in a three-year clinical trial designed to evaluate potential benefits of ABSORB, announced it deployed the device for the first time after FDA approval for commercial use. The recipient was the first in Pennsylvania to receive the device post-trial and the first patient in the in the United States treated with a BVS for heart attack. ABSORB is referred to as a scaffold to indicate its temporary nature, unlike a permanent metallic stent. ABSORB works by restoring blood flow to the heart, keeping the vessel open until it can stay open on its own and then dissolving completely over time. The trial showed that ABSORB performed as well as a drug-coated metallic stent.
"As a physician, it is invigorating to be a part of clinical research that positively impacts our patients in central Pennsylvania," said William Bachinsky, director of cardiovascular research and the cardiac catheterization labs at PinnacleHealth. "By participating in clinical trials at PinnacleHealth, we bring cutting-edge technology to the region years before FDA approval for commercial use."
Source-Newswise