Patients could opt for Proton Pump Inhibitors (PPI) for temporary relief from acidity, suggests new study. The move came from the information that Ranitidine contains carcinogens.

‘Patients with gastroesophageal reflux or acid reflux and acidity could opt for alternative drugs like proton pump inhibitors. More research is required to attribute the blame to ranitidine as the one containing carcinogens.’
Read More..




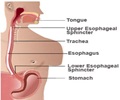
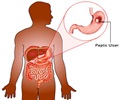
"It has recently been brought to light that ranitidine could be having a cancer causing impurity N-Nitrosodimethylanine (NDMA). The patients who suffer from gastroesophageal reflux, commonly known as acid reflux or acidity, can also be treated with PPI in the place of ranitidine," says Zeenat Ahmed, Senior Consultant, the Department of Internal Medicine at Jaypee Hospital in Noida. Read More..
Some drug regulators in other countries have found it to contain a chemical compound that can cause cancer. "People should not consume these medicines without prescription or doctor's consultation," Ahmed stressed.
On Tuesday, Drugs Controller V.G. Somani issued a directive alerting states about ranitidine and telling them to ask drug manufacturers to take measures for the patient safety.
Niranjan Naik, Director, Surgical Oncology, Fortis Memorial Research Institute (FMRI) in Gurugram, said ranitidine was one of the oldest drugs used for acid peptic disease.
"The present controversy is due an impurity NDMA associated with some brands. It needs further research to attribute the blame to ranitidine, one of the most economical options available for treatment of acid peptic diseases," Naik said.
Advertisement
Advertisement