Three-drug combo improves lung function in most common genetic form of cystic fibrosis, a chronic, progressive, fatal genetic disease that affects the respiratory and digestive systems in children and young adults.
‘Elexacaftor-tezacaftor-ivacaftor combination therapy was highly effective, safe and well tolerated in cystic fibrosis patients with Phe508del CFTR mutation.’
Read More..




Dr. Raksha Jain, Associate Professor of Internal Medicine at UT Southwestern Medical Center, is corresponding author of the NEJM article and an investigator on The Lancet study. Dr. Jain is presenting both studies at the North American Cystic Fibrosis Conference in Nashville this week. Read More..
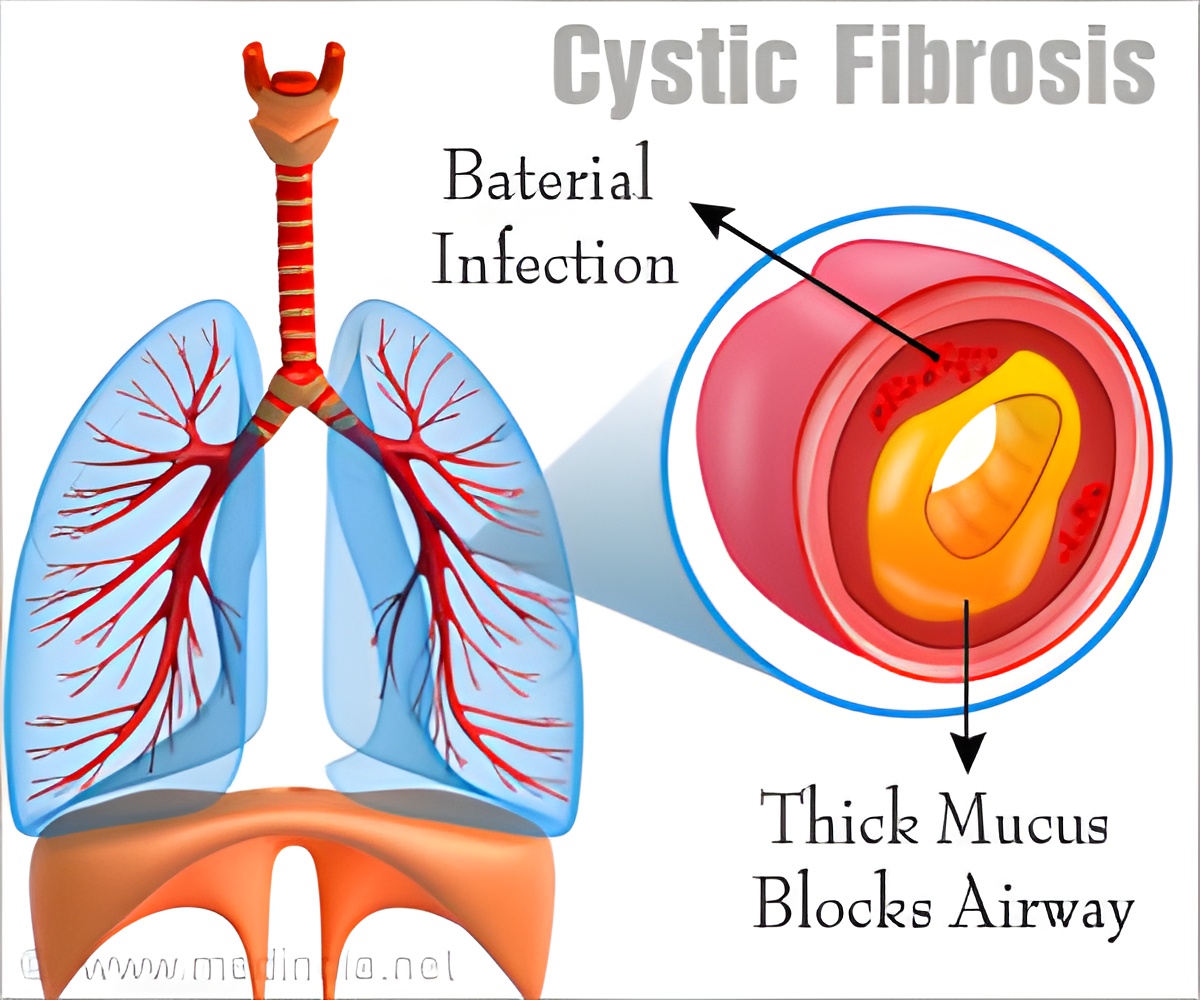
The sweat glands and the reproductive system are also usually involved in CF. Individuals with CF have a shortened lifespan.
"Although there are over a thousand different disease-causing mutations, nearly 90 percent of people with cystic fibrosis have at least one copy of the most common mutation, the Phe508del CFTR allele," Dr. Jain said.
An estimated 80,000 people worldwide are affected by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, she said. People inherit a gene from each parent that encodes the CFTR protein.
"This three-drug combination was highly effective in people with cystic fibrosis who inherited the Phe508del CFTR mutation, improving health outcomes and symptoms," said Dr. Jain, referring to the NEJM study on those with one mutated copy of the gene.
Advertisement
Lung function was measured at four and 24 weeks. Compared with patients receiving a placebo, lung function in the treatment group was significantly improved at four weeks and sustained through week 24.
Advertisement
Excessive amounts of salt via sweating is a hallmark of cystic fibrosis. The treatment group had a lower concentration of salt in their sweat than the placebo group, which demonstrates how this therapy in targeting the underlying cause of the disease, she added.
Adverse events leading to discontinuation occurred in 1 percent of those getting the drug combination. Although the therapy was generally safe and well-tolerated, long-term studies are needed to further understand potential side effects, Dr. Jain said.
"The CF community is working hard to find highly effective therapies for people who are not eligible for this treatment because they don't have the appropriate gene mutation," said Dr. Jain, a Dedman Family Scholar in Clinical Care and Director of the UTSW Adult Cystic Fibrosis Center.
Source-Eurekalert