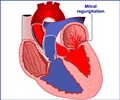
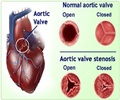
An international study found a drug meant to protect heart during bypass surgery failed to reduce deaths, but complications among bypass surgery patients were much less than reported before.
"We expected about 10 percent of patients were at high risk for complication following coronary artery bypass grafting," said Mark F. Newman, M.D., chairman of the Department of Anesthesiology at Duke and lead author of the study. "But the actual risk was 5 percent. What that means is cardiac surgery has gotten much safer, even for high-risk patients."
Newman said surgical management of patients undergoing coronary artery bypass grafting (CABG) has improved worldwide in the past decade.
The study, which began in 2009, tested a drug called acadesine in a Phase III trial that was one of the largest involving a surgical procedure. The primary objective was to determine whether acadesine would cut the rate of complications from CABG – the most common type of open heart surgery in the United States. The procedure is highly successful in restoring blood flow to the heart caused by blockages, but strokes, ventricular damage and death can result even after successful surgery.
One cause of complications is called ischemia reperfusion injury, which stems from changes that occur to tissue starved of oxygen during the surgery, when blood vessels are clamped to establish the grafts. This period of oxygen deprivation triggers inflammation and cell death once blood flow is restored.
Earlier evidence from smaller studies suggested acadesine, given before, during and after surgery, could offer protection by easing some of the inflammatory responses that kill cells. Most of those studies occurred before 1997, however, when the rate of serious complications was 10 percent or greater.
Advertisement
Still, the 5 percent complication rate was good news, Newman said. It is likely the result of improved surgical methods in recent years, including better anesthesia and advances in surgical and heart-lung machine management.
Advertisement
Newman also said that support for publication of a negative study by the sponsor, Merck, and by the publisher JAMA, is an important step in defining the appropriate therapy for heart surgery patients going forward.
Source-Eurekalert