The investigational Ebola treatment mAb114 is safe, effective and easy to administer in adults, found an early stage clinical trial.
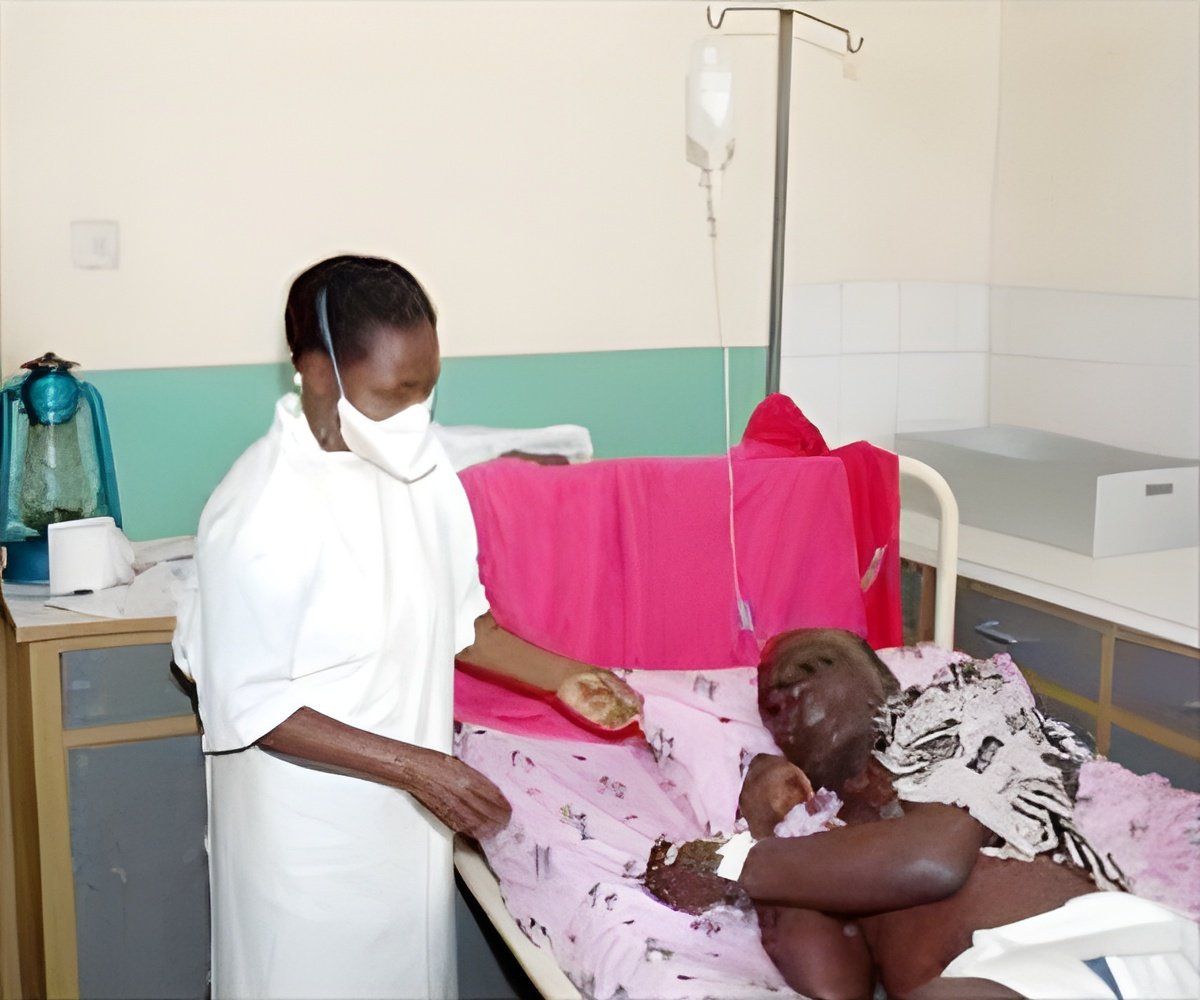
‘The versatility that exists in the monoclonal antibody is advantageous and can be quickly deployed in an Ebola outbreak environment.’





Participants in the Phase 1 clinical trial received a single intravenous infusion of mAb114, administered over approximately 30 minutes. Three participants received a 5 milligram(mg)/kilogram (kg) dose; five participants received a 25 mg/kg dose; and 10 participants received a 50 mg/kg dose. All infusions were well-tolerated. Four participants reported mild side effects, such as discomfort, muscle or joint pain, headache, nausea, and chills in the three days following the infusion. As expected, levels of mAb114 in the blood increased as the dosage was increased. Investigators also observed relatively uniform levels of absorption, distribution, and elimination of mAb114 among participants.
The authors note several advantages for deploying mAb114 in an outbreak setting, including the ease and speed of its administration, and its formulation as a freeze-dried powder that does not require freezer storage. The powder is reconstituted with sterile water and added to saline for administration.
In addition to the ongoing Phase 2/3 clinical trial of mAb114 in the DRC, the VRC is planning to initiate another Phase 1 trial of the investigational treatment in Africa.
Advertisement