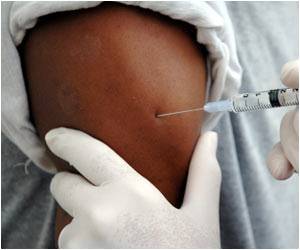
Officials have confirmed that a second clinical trial testing a Canadian-made vaccine against Ebola in humans began on Wednesday.
The latest trial involves trying two doses, a strategy known as prime-boost, on 39 healthy adults to see if it generates a response from their immune systems, the NIH said in a statement.
Earlier this month, the Walter Reed Army Institute of Research began its own test of VSV-ZEBOV as a single dose vaccine at its facility in Maryland.
The vaccine cannot make someone become sick with Ebola.
VSV-ZEBOV is one of two experimental vaccines that the UN health agency said has shown promising results when tested on monkeys.
The other is made by British company GlaxoSmithKline (GSK) and the US National Institute of Allergy and Infectious Diseases (NIAID). That vaccine began human testing in September.
Advertisement
"The need for a vaccine to protect against Ebola infection is urgent," NIAID chief Anthony Fauci said.
Advertisement
There is no approved drug to treat Ebola and no vaccine on the market to prevent it, even though the virus was first discovered in 1976.
The fast-growing outbreak in West Africa, which has killed more than 4,800 people since the start of the year, has pressed global authorities to fast-track vaccine testing.
Source-AFP