Edwards Lifesciences Corporation revealed that it has received the CE Mark in Europe for the use of its valve-in-valve procedures.
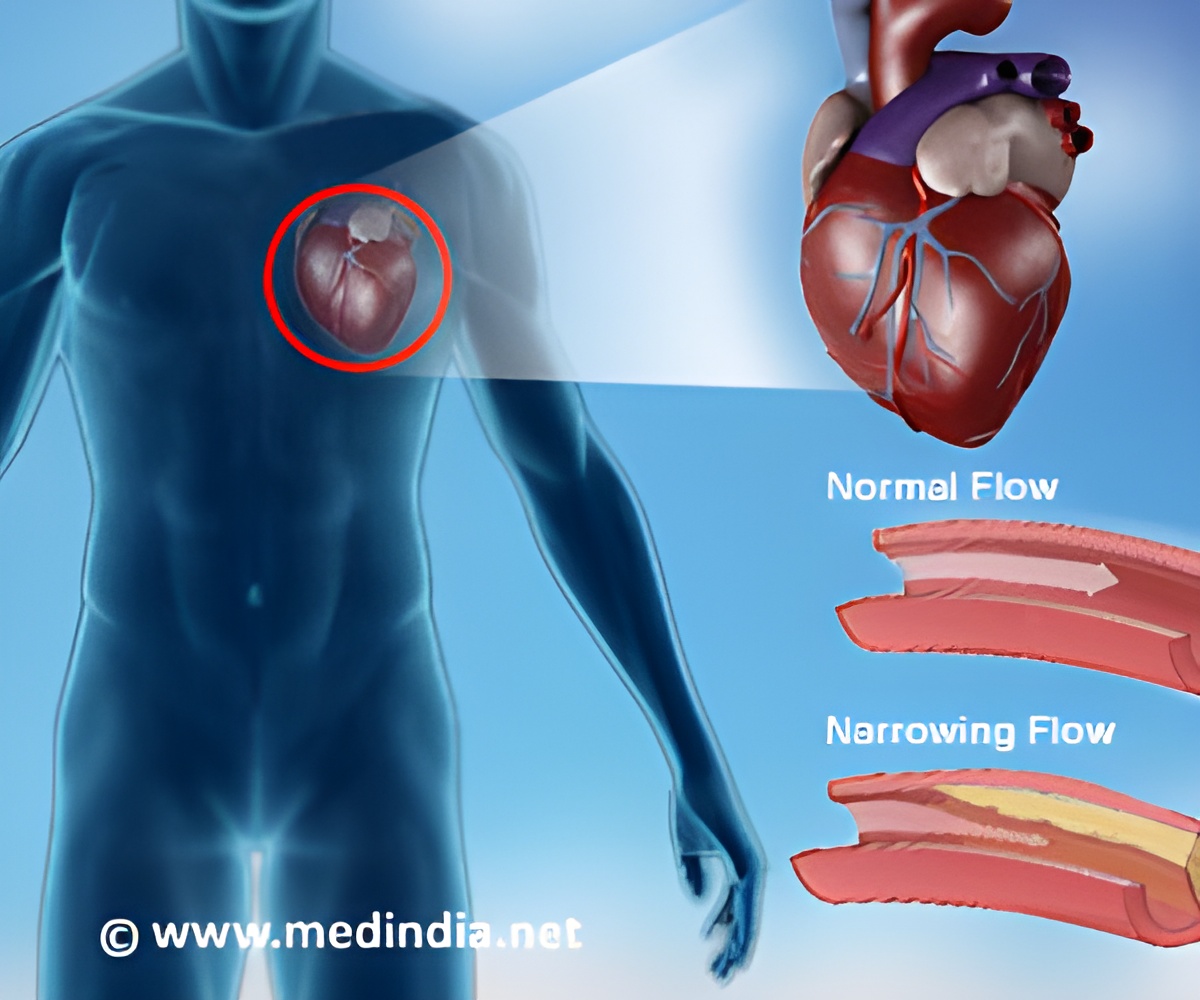
California-based Edwards Lifesciences Corporation revealed that it has received the CE Mark in Europe for the use of its valve-in-valve procedures using the SAPIEN XT transcatheter heart valve as a minimally invasive treatment option for patients who are at a high risk of surgery and who require a replacement of their surgical mitral or aortic valves.
The company revealed that SAPIEN XT valve can reside on top of a previously installed prosthetic valve and is the only device that has been approved for valve-in-valve procedures inside the mitral valve
“The European approval of the SAPIEN XT system for valve-in-valve procedures is a milestone achievement. While this is not a large financial opportunity, it represents an important benefit for patients unable to go through a second open-heart surgery to replace their failing bioprosthetic valves”, the company’s corporate vice president, transcatheter heart valves, Larry Woods said.
Source-Medindia