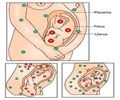
Environmental factors in the womb can predispose on the mother's own offspring as well as the grand-offspring to metabolic disorders like liver disease, a new research reveals.

The findings indicate that in a fetus' reproductive cells, in utero malnutrition causes epigenetic changes that are subsequently transmitted to cells of the next generation. If these findings hold true for humans, what a woman eats while pregnant may have some effects on health and disease in her future grandchildren.
"Current dogma proposes that the vast majority of epigenetic modifications in the sperm and eggs are erased precisely to avoid transmission of environmentally derived changes. But our data suggest that a few environmentally induced epigenetic modifications may be passed and stably maintained in the next generation," says Jiménez-Chillarón. This opens up the possibility that predisposition for some complex diseases might be inherited independently from one's genetic sequence. However, Jiménez-Chillarón notes that it is important not to fall into the temptation of "blaming" one's parents (or even grandparents) for disease: "Our view is that we inherit some predisposition, but it is our own lifestyle that will determine whether inherited risk will truly translate into disease. Hence, a healthy lifestyle is the best way to prevent any potentially inherited or newly acquired obesity or diabetes predisposition."
Source-Eurekalert