Empagliflozin reduced the combined relative risk of cardiovascular death and hospitalization for heart failure patients with and without diabetes by 25 percent.
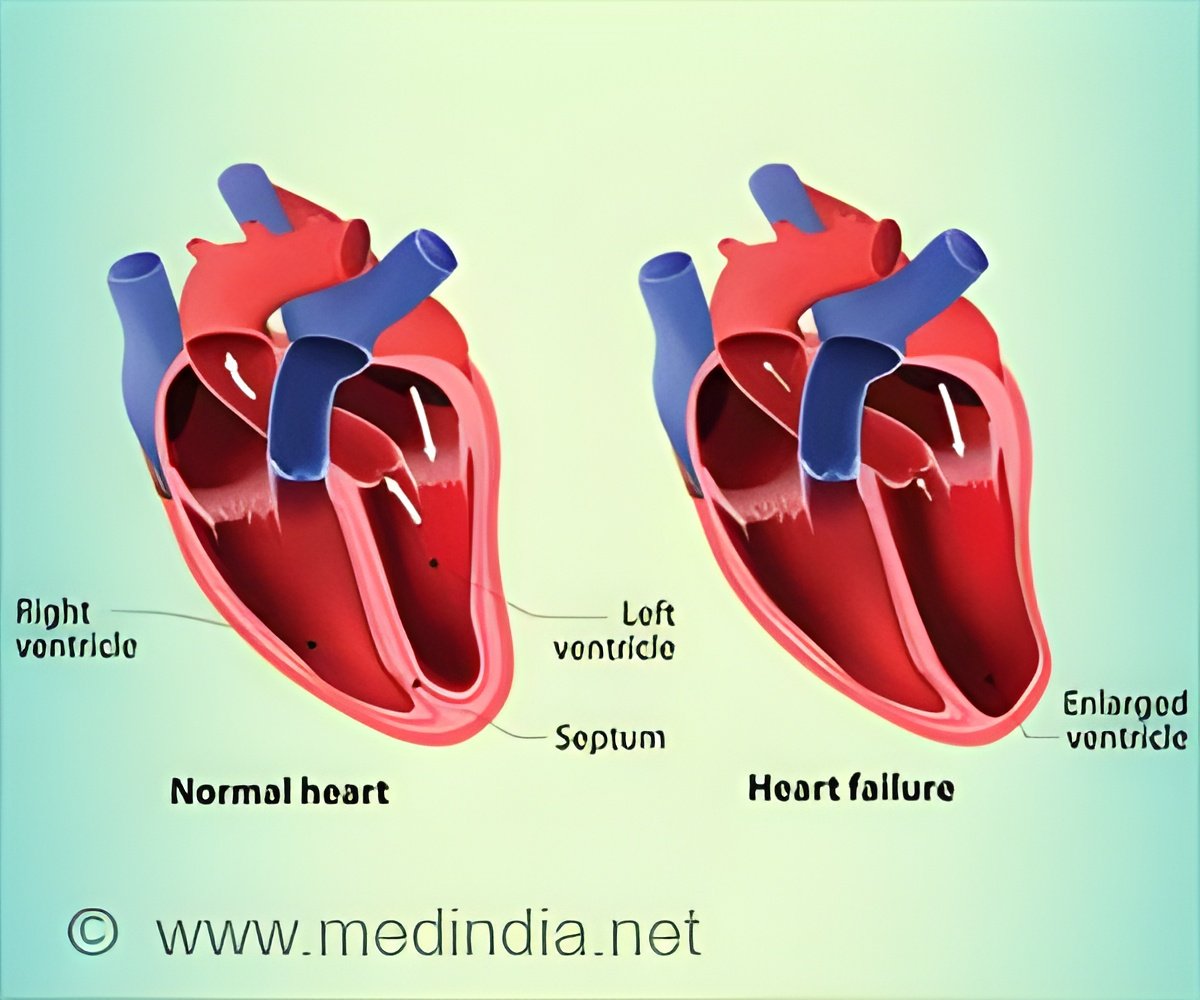
‘Empagliflozin was the first SGLT2 inhibitor to demonstrate a reduction in cardiovascular death in people with type 2 diabetes and established cardiovascular disease, based on the EMPA-REG OUTCOME trial.’
Read More..




The trial evaluated the effect of adding empagliflozin (10 mg) versus placebo to standard of care. The results were presented at the ESC Congress 2020, the annual meeting of the European Society of Cardiology, and published in The New England Journal of Medicine, Boehringer Ingelheim announced.Read More..
The findings from the primary endpoint were consistent in subgroups with and without type 2 diabetes. Key secondary endpoint analyses from the trial demonstrated that empagliflozin reduced the relative risk of first and recurrent hospitalization for heart failure by 30 percent.
Additionally, the rate of decline in eGFR, a measure of kidney function decline, was slower with empagliflozin than with placebo.
"Heart failure is a devastating and debilitating cardiovascular condition. Not only does it limit quality of life, but it is also a progressive disease that requires repeated hospitalizations and is accompanied by a loss in kidney function,” said Milton Packer, M.D., Chair of the Executive Committee for the EMPEROR Program and Distinguished Scholar in Cardiovascular Science at Baylor University Medical Center in Dallas, Texas, U.S.
“Results from the EMPEROR-Reduced trial show that, when given to adults with heart failure with reduced ejection fraction, empagliflozin reduces the number of heart failure hospitalizations while slowing the decline of kidney function. These results are highly statistically significant and clinically important.”
Advertisement
An additional exploratory analysis showed that empagliflozin decreased the relative risk of a composite kidney endpoint*, including end stage kidney disease and a profound loss of kidney function, by 50 percent.
Advertisement
There were no clinically meaningful differences in adverse events including hypovolemia (decreased blood volume), hypotension (low blood pressure), volume depletion (loss of fluids), renal insufficiency (poor kidney function), hyperkalemia (high potassium levels) or hypoglycemic events (low blood sugar) compared with placebo.
Heart failure affects over 60 million people worldwide, with India bearing the burden as one of the largest HF populations worldwide. Heart failure occurs when the heart cannot pump sufficient blood to the rest of the body and is the most common and severe complication of a heart attack.
People with heart failure often experience breathlessness and fatigue, which can severely impact their quality of life. Individuals with heart failure often also have impaired kidney function, which can have a significant negative impact on prognosis.
“Heart failure can have a profound impact on people living with the condition, with the potential of life limiting consequences for the heart and the kidneys”, said Waheed Jamal, M.D., Corporate Vice President and Head of CardioMetabolic Medicine, Boehringer Ingelheim.
"Empagliflozin was the first SGLT2 inhibitor to demonstrate a reduction in cardiovascular death in people with type 2 diabetes and established cardiovascular disease, based on the EMPA-REG OUTCOME® trial.
We continue to break new ground with the EMPEROR-Reduced results, which provide robust evidence that empagliflozin can transform the lives of millions of people through reducing cardiovascular outcomes and slowing the progression of kidney damage in people with heart failure. We look forward to exploring these data further and are planning regulatory submissions for later this year.”
The U.S. Food and Drug Administration (FDA) has granted Fast Track designation to empagliflozin for the reduction of the risk of cardiovascular death and hospitalization for heart failure in people with heart failure. This designation is for the EMPEROR program, which consists of the EMPEROR-Reduced and EMPEROR-Preserved trials.
EMPEROR-Preserved is exploring the effect of empagliflozin on cardiovascular death or hospitalization for heart failure in adults with heart failure with preserved ejection fraction, an area that currently has no approved treatment options. EMPEROR-Preserved results are expected in 2021.
Additionally, the ongoing EMPA-KIDNEY study is evaluating the effect of empagliflozin on the progression of kidney disease and occurrence of cardiovascular death in adults with established chronic kidney disease, with and without diabetes.
The FDA has also granted Fast Track designation to empagliflozin for the treatment of chronic kidney disease, demonstrating the urgent need for new treatment options for people living with the condition worldwide. Results from EMPA-KIDNEY are expected in 2022.
The EMPEROR and EMPA-KIDNEY studies are part of the EMPOWER clinical program, the broadest and most comprehensive of any SGLT2 inhibitor, exploring the impact of empagliflozin on the lives of people across the spectrum of cardio-renal-metabolic conditions.
The program also includes the EMPACT-MI study, which will investigate the effect of empagliflozin on all-cause mortality and hospitalization for heart failure in adults, with and without diabetes, who have had a heart attack , and the EMPULSE study, which is exploring empagliflozin in adults, with and without diabetes, who are hospitalized for acute heart failure and have been stabilized.
Composite exploratory endpoint included chronic dialysis or renal transplant or sustained reduction of ≥ 40% in eGFR (CKD-EPI) or a sustained eGFR < 15 mL/min/1.73m2 (for patients with baseline eGFR ≥ 30) or sustained eGFR < 10 mL/min/1.73m2 (for patients with baseline eGFR < 30 mL/min/1.73m2).
Source-Medindia