Understanding sugar metabolism is more complicated than a simple digestion. Chemotaxis along a chemical gradient aids in the assembly of enzymes like metabolons.
‘Chemotaxis is where molecules migrate along a chemical gradient could be a factor in assembling into enzymes like metabolons.’





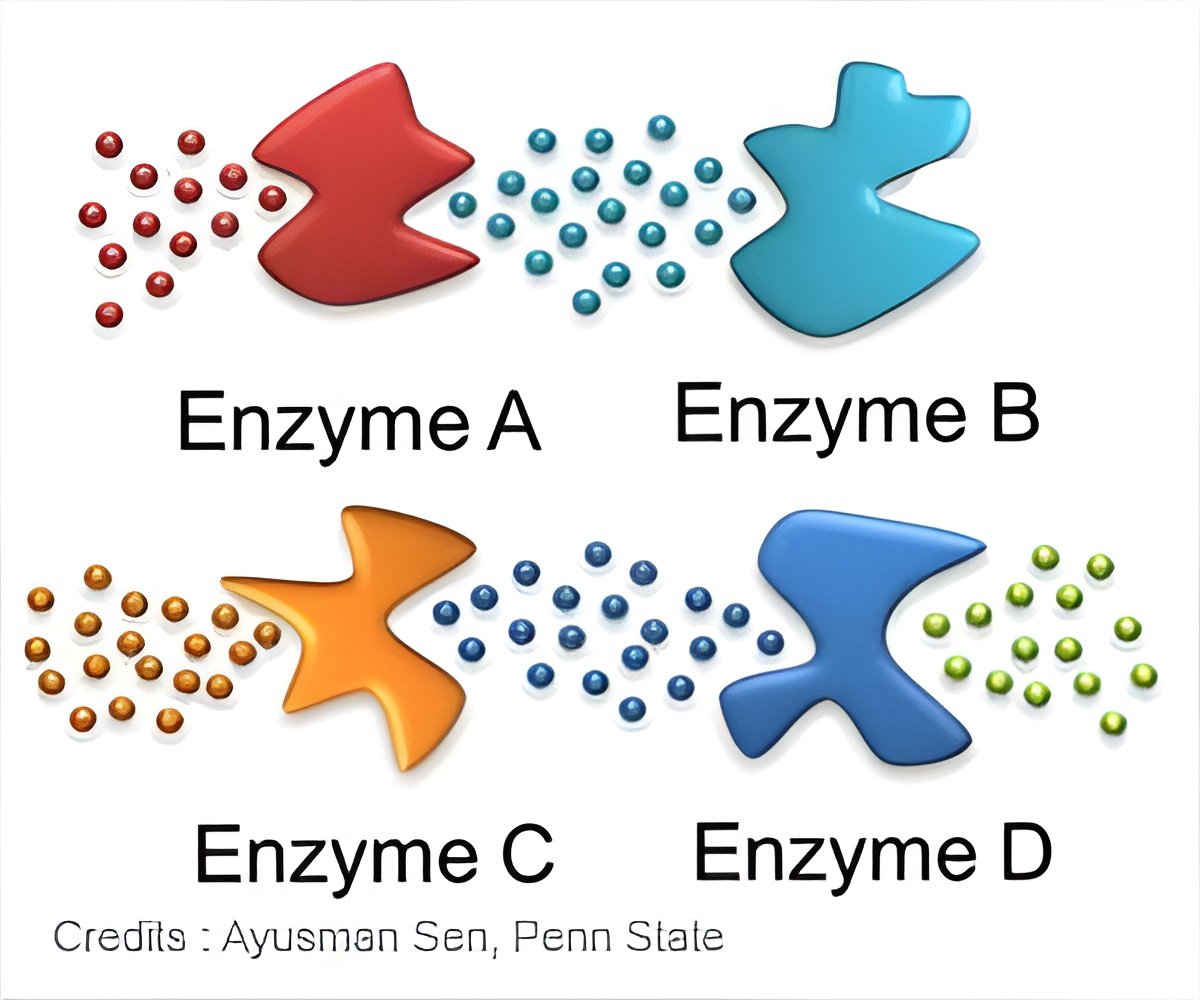
In this cascade, the first enzyme acts on the glucose supplied to the cell and the subsequent enzymes work on successive products. In the process, two adenosine triphosphate molecules ATP are consumed but four are produced. The hydrolysis of ATP powers many cellular processes to maintain the cell's viability. Similar enzyme cascades are responsible for many metabolic processes in the body.Enzymes that participate in such reaction pathways have in some cases been shown to form intracellular, reversible complexes termed metabolons by Paul Srere (deceased), University of Texas Southwestern Medical School. Having the enzymes in proximity to one another facilitates the series of reactions they catalyze.
One such example is the purinosome discovered in Evan Pugh University Professor and Eberly Chair in Chemistry Stephan J. Benkovic's Lab at Penn State that consists of six enzymes involved in the biosynthesis of purines.
The researchers asked whether one of the factors contributing to metabolon formation could be a gradient of chemicals generated by the participating enzymes. They report their results in the issue of Nature Chemistry.
"We discovered some time ago that simple catalyst molecules such as enzymes will also chemotax up the gradient of a reactant," said Ayusman Sen, distinguished professor of chemistry, Penn State. "They move toward higher and higher concentrations of reactant."
Advertisement
"All living things chemotax," said Sen. "If you are hungry and suddenly smell French fries, you will try to walk toward the fries. If the smell decreases, you will randomly turn to try to find the higher concentration of French-fry odor molecules until you are at the French-fry counter."
Advertisement
They looked at three cases - the normal reaction where hexokinase phosphorylates glucose; the reaction of hexokinase with mannose, a sugar that binds more strongly but has a slower reaction rate; and finally with L-glucose, a form of glucose that is not used by hexokinase. The phosphorylation requires ATP. In the presence of phosphoglucose isomerase - the second enzyme and phosphofructokinase - the third enzyme, the reactant for aldolase is produced.
The researchers observed that the aldolase moves towards the hexokinase in their flow experiment, revealing that aldolase was chemotaxing up the reactant gradient produced by the functioning of the first three enzymes in the pathway. The chemotaxis was greatest with D-glucose, less with mannose and not observed with L-glucose.
Theoretical modeling of the enzyme movement qualitatively predicted the extent of enzyme movement.
The researchers also looked at whether chemotaxis of enzymes would occur in a model of the exceptionally crowded intracellular environment. They added a large molecular weight substance to simulate such crowding. Chemotaxis still occurred, but at a slower rate.
"Chemotaxis along a chemical gradient could be a factor in assembly of enzyme clusters such as metabolons," said Benkovic. "Other factors, such as noncovalent interactions would still be expected to contribute."
The resolution of the research instrument, however, was insufficient to demonstrate in this case that the four enzymes were assembling into a metabolon. The researchers observed the formation of large aggregates of enzymes, but could not demonstrate they were functioning metabolons.
Source-Eurekalert