Global clinical trial designed in collaboration with VCU successfully tested a drug therapy to treat NASH, a chronic liver disease and the fastest-growing reason for liver transplantation in the U.S.

‘Obeticholic acid, the new investigational drug submitted for FDA approval, is a potent, modified version of bile acid that plays a vital role in metabolism.’
Read More..




According to the National Institutes of Health, between 7 million and 30 million U.S. adults suffer from NASH, a severe form of fatty liver disease unrelated to alcohol abuse but closely related to obesity and Type 2 diabetes. Read More..
"NASH is definitely a modern lifestyle disease and on its way to becoming the leading cause of liver transplantation in the U.S. as early as 2020," said Arun Sanyal, M.D., the senior and corresponding author of the manuscript, and a professor in the Department of Internal Medicine in the VCU School of Medicine. "We hope that this landmark study will lead to the first approved drug therapy for NASH."
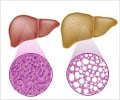
In patients suffering from NASH, the buildup of fat in the liver damages cells and causes inflammation. For many, NASH can progress to include scarring, called fibrosis, which prevents the liver from functioning properly.
In severe cases, the condition can lead to liver cancer, liver failure or death. The REGENERATE study assesses the safety and efficacy of obeticholic acid in patients with liver fibrosis due to NASH.
"The treatment tested can reverse the course of NASH," Sanyal said. "Obeticholic acid showed an ability to successfully reverse liver scarring in patients most at risk for irreversible liver damage when evaluated 18 months into the study. The study will also follow the patients long term to see if these changes seen on a liver biopsy translate into improved clinical outcomes."
Advertisement
He currently is engaged in studies to develop such diagnostics. In the U.S. alone, the prevalence of NASH is expected to increase by 63% between 2015 and 2030.
Advertisement
The scientific understanding of how bile acids enhance glucose disposal, which is a critical element of a condition called insulin resistance, led to a successful, federally funded Phase II FLINT trial in 2015, paving the way for the REGENERATE trial.
"Obeticholic acid, the new investigational drug submitted for FDA approval, is in essence a more potent, chemically modified version of a naturally occurring bile acid that plays a key role in our metabolism and also has anti-fibrotic effects," Sanyal said.
Source-Eurekalert