Researchers have developed a new, experimental vaccine that protects against deadly pneumonia.

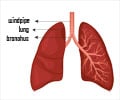
The National Foundation for Infectious Diseases estimates that 175,000 people are hospitalized with pneumococcal pneumonia in the United States each year. In addition to pneumonia, pneumococcus causes 34,500 bloodstream infections and 2,200 cases of meningitis annually. It is responsible for more deaths in the United States 4,800 a year than any other vaccine-preventable disease. It poses a particular problem in the developing world, where it is estimated to cause more than one million deaths in children each year, according to the World Health Organization. Details of the new vaccine, which was tested in an animal model, are reported in a paper published today in the Journal of Infectious Diseases.
A pediatric vaccine has dramatically reduced the incidence of pneumococcal disease in children and adults, both by protecting vaccinated children and by reducing person-to-person transmission of the bacterium to others a phenomenon known as herd immunity.
The pediatric vaccine is a great victory for modern medicine, but it doesn't cover all strains of disease-causing pneumococcus some of which have recently emerged and are very virulent, said Dr. Pirofski. This problem, coupled with the fact that herd immunity doesn't protect immunocompromised patients as effectively as people with normal immunity, led us to look for a better vaccine.
The researchers focused on developing a vaccine against serotype 3 a pneumococcal strain that was not included in the pediatric vaccine used for the past decade and that has emerged as a cause of serious pneumonia in adults and children. Serotype 3 can trigger inflammation so overwhelming that it can result in very severe disease or even death.
The goal of this study was to produce a vaccine consisting of a live, attenuated (weakened) version of serotype 3 S. pneumoniae. To create their vaccine, the researchers focused on the serotype 3 gene that codes for pneumolysin, a toxin produced by all pneumococcal strains. The researchers replaced this gene with a synthetic version that they hoped would reduce the amount of toxin produced.
Our idea was to design a live vaccine that would stimulate the immune system sufficiently to ward off disease but wouldn't lead to the severely damaging inflammatory response that this strain can cause,said lead author J. Robert Coleman, Ph.D., a postdoctoral fellow in microbiology & immunology at Einstein, who helped develop the gene-modification technique, known as synthetic gene customization, while a graduate student at Stony Brook University.
Advertisement
Altering the pneumolysin gene in the seroptype 3 bacteria resulted in less pneumolysin toxin produced in vitro. When mice were injected with either attenuated or unattenuated serotype 3 bacteria, mice receiving the attenuated strain developed an inflammatory response much weaker than was observed in mice receiving the unattenuated serotype 3 strain. Most important, of the five mice injected with the attenuated strain, four survived a subsequent challenge from the highly virulent unattenuated serotype 3 strain, which was lethal in five of five unvaccinated, control mice.
Advertisement
The paper, "Designed reduction of Streptococcus pneumoniae pathogenicity via synthetic changes in virulence factor codon-pair bias," was published in the February 22 online edition of the Journal of Infectious Diseases. This research was funded by the National Institutes of Health and the National Institute of Allergy and Infectious Diseases.
Source-Newswise