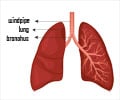
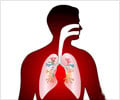
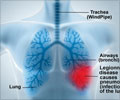
Pfizer’s pneumonia vaccine for children has been backed by an FDA advisory committee to be used in treating diseases caused by pneumococcus bacteria in adults.
The health regulator is said to be considering placing the vaccine on an accelerated approval process and will reach a decision soon on whether to approve the vaccine to be used in adults over 50 years of age.
The vaccine, which was approved in October 2010 for preventing diseases caused by S. pneumoniae serotypes in children, generated over $3.7 billion in sales worldwide and Pfizer revealed that it could generate an additional $1.5 billion annually if it is approved for adults.
Source-Medindia