Popular osteoporosis drugs known as bisphosphonates are being re-evaluated by the advisory panel of the US Food and Drug Administration
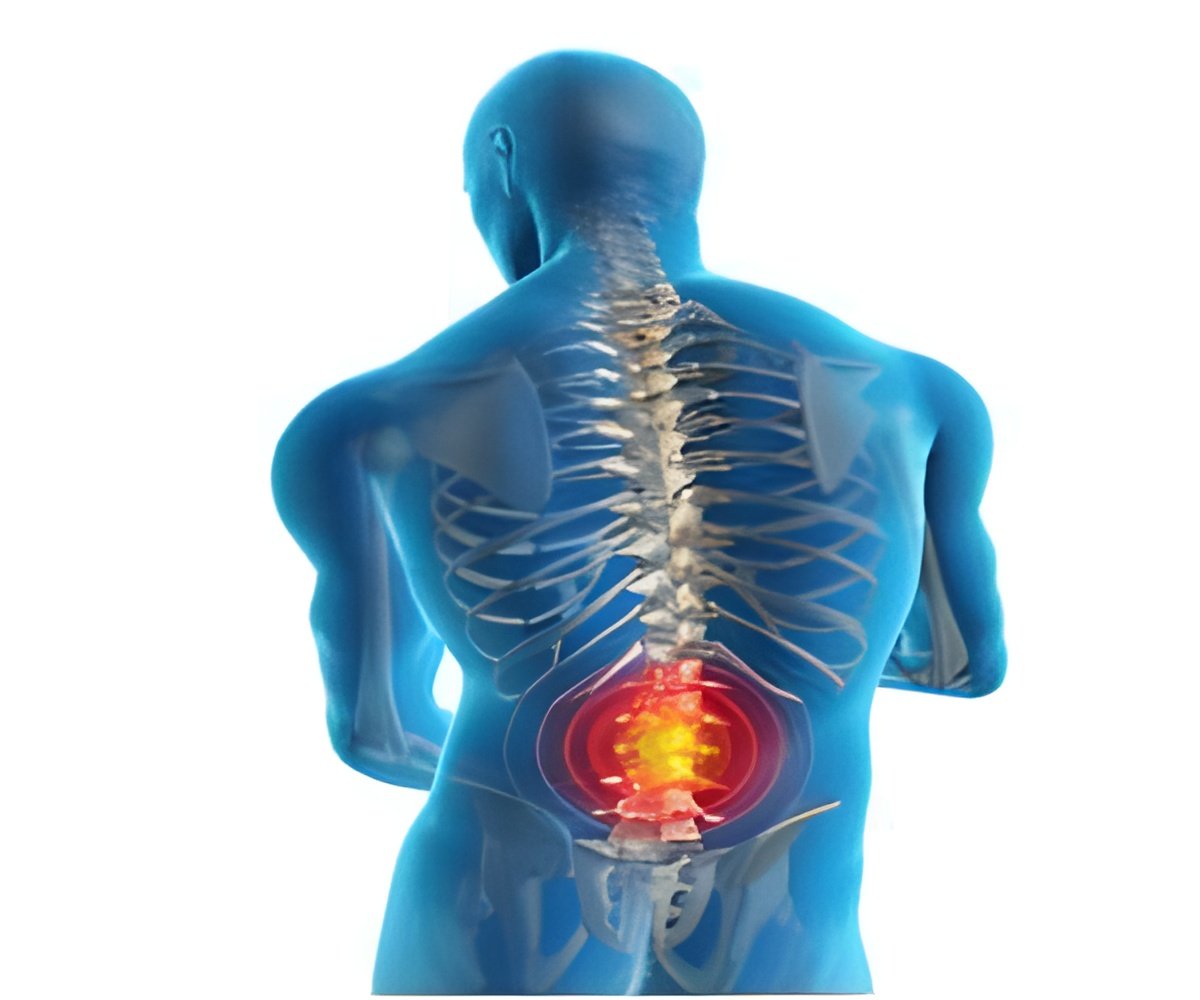
Popular osteoporosis drugs known as bisphosphonates are being re-evaluated by the advisory panel of the US Food and Drug Administration after recent studies linked this class of drugs with brittle bones in thighs and the jaw.
A study conducted by the American Society of Bone and Mineral Research last September revealed that bisphosphonates increased the risk of rare femur fracture among patients suffering from osteoporosis. The researchers observed 310 cases of such fractures and found that more than 94 percent of them had taken the drugs.
The researchers suggested that the FDA should consider their study and change the drugs’ labeling to display warnings of such risks.
One of the popular bisphosphonate drugs, Fosamax, has also been linked with osteonecrosis of the jaw, though its manufacturer Merck said that the studies lack any clinical evidence.
Source-Medindia