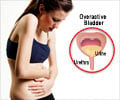
FDA has approved botox injections for the treatment of overactive bladder.

"Urinary incontinence associated with neurologic conditions can be difficult to manage," said George Benson, deputy director of FDA's division of Reproductive and Urologic Products.
"Botox offers another treatment option for these patients."
The new method allows a physician to inject Botox into a patient's bladder, where it relaxes the muscles and allows more urine to be stored.
Clinical studies showed such injections could decrease episodes of urinary incontinence for a period of nine months.
Botox, which is marketed by the California-based Allergan, is also approved for treatment of chronic migraines, severe underarm sweating, eyelid twitching and certain kinds of muscle stiffness, the FDA said.
Advertisement
Source-AFP