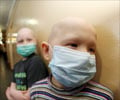
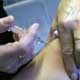
The US Food and Drug Administration said Tuesday it has approved a Canadian-made shot against swine flu.
The US Food and Drug Administration said Tuesday it has approved a Canadian-made shot against swine flu.
The vaccine is the fifth approved by the FDA for use in the United States, where a massive campaign to vaccinate tens of millions of Americans against swine flu has come up against a severe shortage of vaccine.Fewer than half of the 160 million doses that health officials had originally said would be available by the end of October have been delivered.
The shortfall has been blamed in part on the outdated method used to produce vaccine, which relies on growing a seed stock of the virus in chicken eggs.
Like the other four vaccines approved by the FDA for use in the United States, the new vaccine, made by ID Biomedical of Quebec, is manufactured "using the established, licensed egg-based manufacturing process used for producing seasonal flu vaccine," the US food and drug regulator said.
The vaccine will be produced in multi-dose vials and will contain the preservative thimerosal, which contains mercury.
Pregnant women, who are one of five groups at heightened risk of developing severe complications and dying from swine flu, have to have the injectable form of the pandemic H1N1 vaccine because it is made with killed virus.
Advertisement
Swine flu vaccine was tested on pregnant women in the United States, but the vaccine that was tested did not contain thimerosal.
Advertisement
SRM