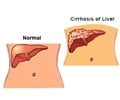
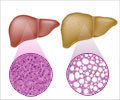
Hereditary tyrosinemia type 1( HT-1) is a genetic metabolic disorder that causes progressive liver failure and liver cancer in young children. It's a
Hereditary tyrosinemia type 1( HT-1) is a genetic metabolic disorder that causes progressive liver failure and liver cancer in young children. It's a very rare disease. It is not among the metabolic diseases that all newborns are tested for at birth, so often infants die undiagnosed. This is an absolutely fatal disease until now.
Children born with such a very rare but very lethal liver disease have won the first drug that promises to help them live years longer. The drug, named Orfadin, amazingly is a failed weed killer that Swedish scientists discovered could fight a disease called hereditary tyrosinemia."This is a real breakthrough drug," said Dr. Marlene Haffner of the U.S. Food and Drug Administration. She said scientists once referred to the drug as "Lazarene, because these kids were so sick and then they were well."
For babies who are diagnosed, the only treatment is a special low-protein diet. Orfadin may significantly improve those dismal statistics. It works by blocking formation of the liver-destroying toxins. The drug's cost depends on the dose, which varies with the child's size: $12,000 a year for an infant, up to $60,000 a year for an older child.