The FDA has approved the first human clinical trials for pig kidney transplants, marking a key step in addressing the organ shortage.
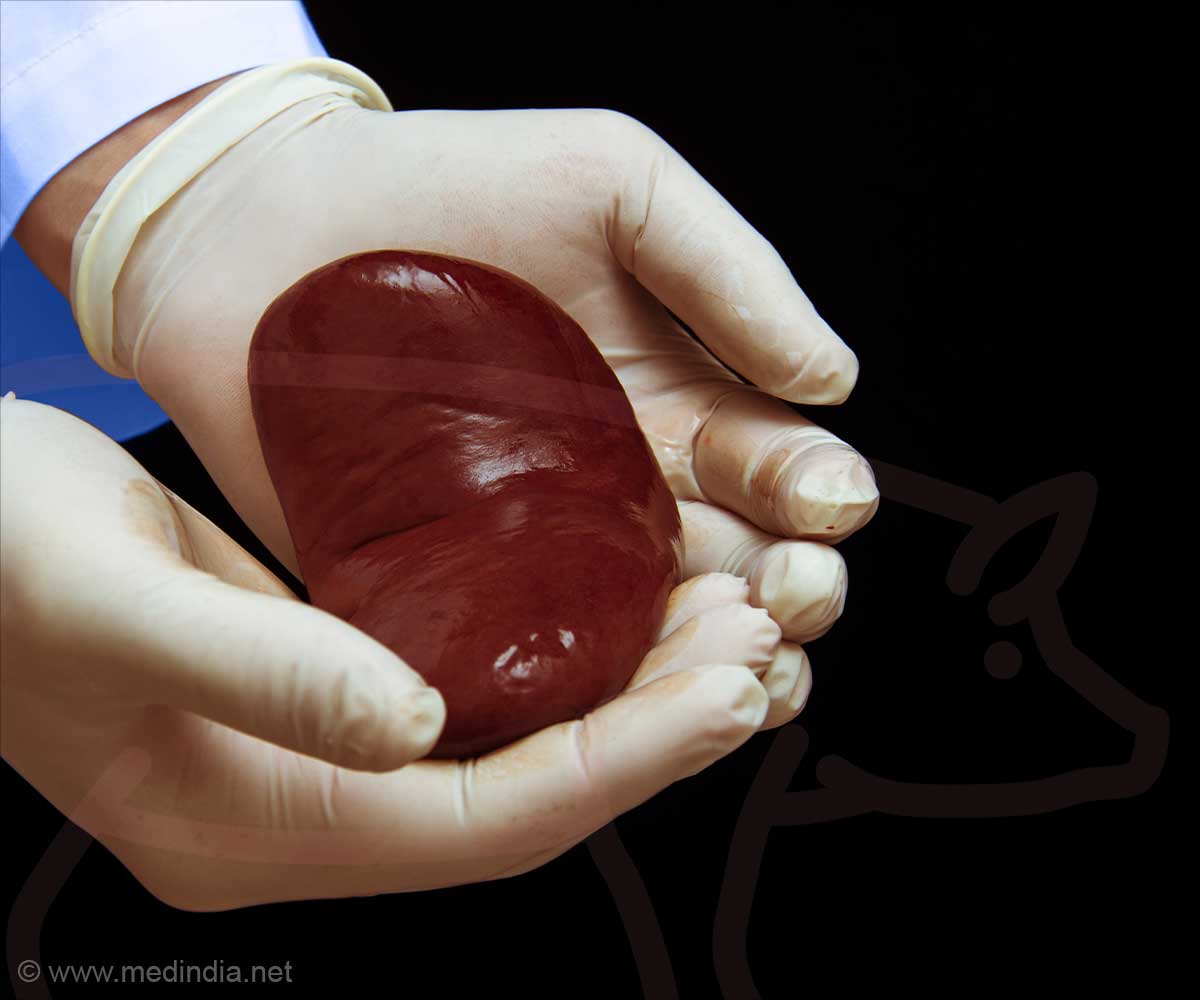
FDA Greenlights Clinical Trials For Groundbreaking Pig Kidney Transplants In Humans
Go to source). With more than 550,000 Americans suffering from kidney failure and nearly 100,000 on the transplant waiting list, the demand for donor kidneys vastly outstrips supply. Only about 25,000 kidney transplants are performed each year, leaving many patients waiting for years or, sadly, dying before receiving a suitable organ. The approval of these trials brings hope to thousands, as genetically modified pig kidneys could provide a scalable, long-term solution.
‘Did You Know?
On average, a kidney transplant from a living donor lasts about 15 to 20 years, and a kidney from a deceased donor lasts 8 to 12 years. #medindia #kidney #transplant’





On average, a kidney transplant from a living donor lasts about 15 to 20 years, and a kidney from a deceased donor lasts 8 to 12 years. #medindia #kidney #transplant’
Advertisement
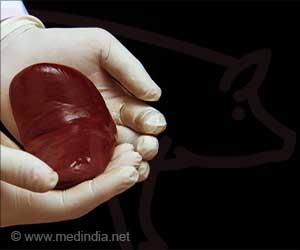
Pigs as Viable Alternatives for Organ Transplantation
Pigs have long been seen as a promising source for xenotransplantation because their kidneys closely resemble human kidneys in size and function. Both United Therapeutics and eGenesis have made extensive genetic modifications to pigs to improve compatibility and minimize the risk of organ rejection. This strategy aims to provide a viable long-term solution for patients who have run out of traditional transplant options.United Therapeutics has proposed a phased trial plan, starting with six patients who have been on dialysis for at least six months and are otherwise stable. If the initial results are successful, the study will be expanded to include up to 50 participants.
Similarly, eGenesis will begin with three patients, spacing out procedures to carefully monitor each case before progressing. The trials are set to start by mid-2025, giving researchers enough time to evaluate safety, effectiveness, and potential complications.
Each participant will go through a thorough 24-week monitoring period, followed by lifelong medical assessments to track long-term outcomes. A key concern is the potential risk of infections being transmitted from pigs to humans, which will require close monitoring and strict medical protocols.
Advertisement
Genetic Modifications to Prevent Immune Rejection
One of the main challenges in xenotransplantation is preventing organ rejection by the human immune system. To tackle this, both companies have used advanced genetic modifications. United Therapeutics’ pigs have undergone 10 gene edits, including adding six human genes and removing four porcine genes associated with immune rejection.eGenesis has adopted a more aggressive strategy, making 69 genetic edits, mostly aimed at deactivating viruses that could pose a threat to human recipients. These modifications are essential to ensure that the transplanted pig kidneys function properly in human bodies without causing severe immune reactions.
Ethical and Safety Concerns in Xenotransplantation
While this medical breakthrough offers hope, it also raises ethical and safety concerns. A major issue is the risk of cross-species infections, as some viruses naturally found in pigs could be life-threatening if transmitted to humans.Additionally, the issue of informed consent has been debated, as many people on the transplant waiting list are in desperate situations. Some experts argue that these patients might feel pressured to accept an experimental procedure, despite the uncertain long-term risks.
There are also economic concerns, as it's still unclear how much these transplants will cost and whether insurance will cover them. If successful, this technology could transform healthcare, but access and affordability will play a crucial role in determining its real-world impact.
Careful Monitoring and Analysis of Each Transplant
With clinical trials set to begin in mid-2025, researchers will take a careful approach, thoroughly analyzing each transplant’s outcome before moving to the next patient. eGenesis has planned a six-month gap between its first two transplants and a three-month gap before proceeding with the third.Even if the trials show positive results, widespread adoption of pig kidney transplants may take several years due to regulatory approvals, cost evaluations, and long-term safety assessments. However, this milestone marks the start of a new era in organ transplantation—one that could save countless lives and transform the future of medicine. The world will be closely watching as science advances toward solving the global organ shortage crisis through biotechnology and genetic engineering.
Reference:
- FDA Greenlights Clinical Trials For Groundbreaking Pig Kidney Transplants In Humans - (https://www.onlymyhealth.com/fda-approves-clinical-trials-for-pig-kidney-transplants-in-humans-12977825129)
Source-Eurekalert