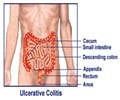
FDA has approved Abbott Laboratories’ rheumatoid arthritis drug Humira for treating moderate to severe ulcerative colitis.
“Each patient with ulcerative colitis experiences the disease differently, and treatment must be adjusted to meet each individual's needs. Today's approval provides an important new treatment option for patients who have had an inadequate response to conventional therapy”, FDA’s Division of Gastroenterology and Inborn Errors Products said.
Humira is Abbott Laboratories’ most successful drug and accounts for annual sales of over $8 billion with analysts predicting that the latest approval by the FDA could generate another $500 million in sales.
Source-Medindia