FDA approves Journavx, a non-opioid medication for acute pain, targeting sodium channels to block pain signals, reducing risks of addiction to opioid painkillers.
FDA Approves Novel Non-Opioid Treatment for Moderate to Severe Acute Pain
Go to source).
What is Journavx?
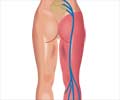
Traditional pain relief medication often relies on opioids by interacting with the body’s opioid receptors. Journavx works by targeting a specific pain-signaling pathway in the body. It affects sodium channels in the peripheral nervous system, that send pain signals to the brain. The drug eases pain by blocking these signals before they reach the brain.‘Did you know?
About six million people struggle with #opioid drug addiction worldwide. #painkiller #painrelief #medindia ’





Pain is an unpleasant sensory and emotional experience due to injury, surgery, or other health conditions. Generally, painkillers are used to treat acute pain (short-term pain that occurs after surgery or trauma) which can sometimes contain opioids. Opioids, while effective, come with the risk of addiction and other adverse side effects.About six million people struggle with #opioid drug addiction worldwide. #painkiller #painrelief #medindia ’
With Journavx, doctors now have a new option to offer patients who need help with acute pain, without the risks associated with opioid use.
Efficacy and Side Effects of Journavx
The efficacy of Journavx was tested in two clinical trials with patients recovering after abdominoplasty and bunionectomy. In these studies, patients who took Journavx experienced significantly less pain than those who were given a placebo. All participants were allowed to take ibuprofen if they couldn’t manage pain, further confirming the benefits of Journavx.The most common side effects of Journavx seen in the trials were itching, muscle spasms, rash, and an increase in a blood enzyme called creatine phosphokinase. Doctors will need to be cautious about giving this drug to patients who are also taking certain other medications like CYP3A inhibitors. Patients are also advised to avoid grapefruit products while taking Journavx, as they can interfere with the efficacy of the drug.
Reference:
- FDA Approves Novel Non-Opioid Treatment for Moderate to Severe Acute Pain - (https://www.fda.gov/news-events/press-announcements/fda-approves-novel-non-opioid-treatment-moderate-severe-acute-pain)
Advertisement