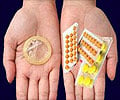
Earlier this week, the Female Health Company (FHC) announced approval from the US Food and Drug Administration (FDA) for the company's Female Condom (FC2).
Earlier this week, the Female Health Company (FHC) announced approval from the US Food and Drug Administration (FDA) for the company's Female Condom (FC2), a woman-initiated barrier method that helps to protect against sexually transmitted infections (STIs), HIV/AIDS, and unintended pregnancy.
FHC's lower-cost second generation female condom will now be sold at 30 per cent less than the earlier version of female condom.FHC's first-generation Female Condom (FC1) originally received FDA approval for distribution in the United States in 1993. FC1 is also included in the World Health Organization's (WHO) essential products list for distribution by United Nations (UN) agencies. Since its approval, 165 million FC1 female condoms have been distributed in 142 countries.
With microbicides still in different stages of the research pipeline, the female condom is currently the only method available to prevent HIV infection and unintended pregnancy that is designed for women's initiation. FDA approval of the FC2 is significant since the new product will sell for about 30% less than its predecessor, the FC1.
Female condoms have been relatively expensive in many parts of the world, due to a constellation of factors including manufacturing costs, bulk purchasing, and government and donor investment. Reduction in manufacturing costs, therefore, is one of many important avenues for making the new female condom more affordable and accessible to women and men in the US and internationally.
FHC has succeeded in reducing FC2's cost through the introduction of a new material and a different manufacturing process. FC1 is made from polyurethane and involves a labor-intensive manufacturing process, while FC2, which looks very similar to FC1, is made from a proprietary nitrile polymer that allows it to be manufactured using a highly automated process. Studies have shown that FC2 performs in a comparable manner to FC1.
Data on FC2 have been reviewed and approved by other regulatory agencies, including the European Union, WHO, and agencies in India and Brazil. In 2006, the World Health Organization (WHO), based on its own review of the scientific data, agreed that FC2 performs in the same manner as FC1 and cleared FC2 for purchase by UN agencies. Since then, over 23 million FC2 Female Condoms have been distributed in 77 countries. FDA approval of FC2 will allow USAID to procure the second-generation female condom at a lower unit cost for US-funded prevention programs around the world.
"We join women around the world in applauding the FDA's swift action to approve the FC2 female condom," stated Serra Sippel, executive director of the Center for Health and Gender Equity, in a press statement. "The HIV pandemic among women requires increased investment in woman-centered prevention options, and FC2 approval is an important step forward in putting the power of prevention in women's hands."
Advocates are now calling on the US government to react quickly to ensure rapid expansion of female condom distribution and programming, and have support from US law. In the reauthorization legislation for the President's Emergency Plan for AIDS Relief (PEPFAR) in 2008, Congress explicitly mentioned female and male condoms, emphasizing the importance of increasing availability and access to these commodities and ensuring consistent and correct use as essential for HIV/AIDS prevention efforts.
"We praise Congress for including specific references to female condoms, as both male and female condoms are safe and effective HIV prevention tools that are available to women and men today. We now look to the next leader of the Office of the Global AIDS Coordinator to ensure that female condoms are truly available, accessible and well-programmed for women and men worldwide," said Serra Sippel in the press statement.
The United States Agency for International Development (USAID) plans to phase out procurement of the FC1 upon FDA approval of the FC2, according to Saving Lives Now, a report by the Center for Health and Gender Equity (CHANGE). This means that potentially more female condoms can be procured, distributed and programmed overseas due to lower costs.
Despite their many benefits, female condoms account for only 0.2% of the world's total condom supply and make up only 1.6% of US international condom shipments. It is unclear how long it will take before the FC2 is distributed through US-funded HIV prevention programs overseas.
Contributed By: Bobby Ramakant
Source-Medindia
SRM