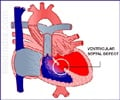
Medtronic said that it won pre-market approval from the FDA for its Melody transcatheter pulmonary valve.
An HDE is intended for devices that will be used in fewer than 4,000 U.S. patients per year; if a device has been demonstrated to have reasonable safety and probable benefit, it doesn't have to be shown to be clinically effective to be approved via this pathway.
In order to make the transition from HDE to PMA approval, Medtronic conducted three clinical trials of the device in a total of 310 patients. In that testing, about 98% of patients did not require open-heart surgery after one year with the Melody TPV implanted. Even after 5 years, 91% of patients with the implant in one of the trials didn't need open-heart surgery.
"The Melody valve has been a reliable option for patients suffering from CHD, and these data reinforce its strong performance since it was first introduced. This approval underscores the valve's importance in treating this small patient population, who over their lifetime will face several open-heart surgeries," said Dr. William Hellenbrand, chief of pediatric cardiology at the Yale School of Medicine and a principal investigator for the device.
Source-Medindia