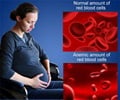
The US Food and Drug Administration confirmed that it has approved a drug for treating morning sickness that was initially pulled off from the market.
Diclegis is a generic version of the drug Bendectin, which had been withdrawn from the market following claims, which have since been debunked, that it increased the risk of birth defects.
Bendectin was manufactured by Merrell Dow who said that it had pulled the drug not because of any pressure from the FDA or because it was ineffective or posed a danger. Instead the company said that it was forced with withdraw the drug as it did not have the financial capacity to defend itself in courts. Diclegis is also made up of doxylamine succinate and pyroxidine hydrochloride and will be available as a single pill, which will be given only on presenting a prescription.
Source-Medindia